A) strongest/shortest
B) strongest/longest
C) weakest/longest
D) weakest/shortest
E) intermediate in both strength and length
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the table of bond dissociation energies, the ΔH for the following gas-phase reaction is __________ kJ. 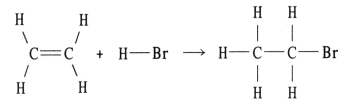
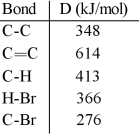
A) 291
B) 2017
C) -57
D) -356
E) -291
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formal charge on sulfur in SO42- is __________, where the Lewis structure of the ion is: 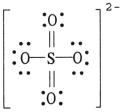
A) -2
B) 0
C) +2
D) +4
E) -4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the Lewis structure of HCO3-, the formal charge on H is __________ and the formal charge on C is __________.
A) -1, -1
B) 0, 0
C) 0, -1
D) +1, -1
E) -1, +1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxidation number of phosphorus in PF3 is __________.
A) -2
B) +1
C) +3
D) +2
E) -3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the table of average bond energies below, the ΔH for the reaction is __________ kJ. 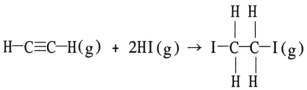 Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
A) +160
B) -160
C) -217
D) -63
E) +63
Correct Answer

verified
Correct Answer
verified
Short Answer
Which halogen, bromine or iodine, will form the more polar bond with phosphorus?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the bonds C-N, C-N, and C≡N, the C-N bond is __________.
A) strongest/shortest
B) strongest/longest
C) weakest/shortest
D) weakest/longest
E) intermediate in both strength and length
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the electronegativities below, which covalent single bond is most polar? Element: H C N O Electronegativity: 2.1 2.5 3.0 3.5
A) C-H
B) N-H
C) O-H
D) O-C
E) O-N
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the table of bond dissociation energies, the ΔH for the following gas-phase reaction is __________ kJ. 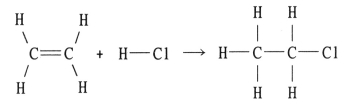
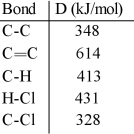
A) -44
B) 38
C) 304
D) 2134
E) -38
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the Lewis structure of ClF, the formal charge on Cl is __________ and the formal charge on F is __________.
A) -1, -1
B) 0, 0
C) 0, -1
D) +1, -1
E) -1, +1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would have to gain two electrons in order to achieve a noble gas electron configuration? O Sr Na Se Br
A) Br
B) Sr
C) Na
D) O, Se
E) Sr, O, Se
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the electron configuration for the Fe2+ ion?
A) [Ar]4s03d6
B) [Ar]4s23d4
C) [Ar]4s03d8
D) [Ar]4s23d8
E) [Ar]4s63d2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Lewis structure of N2H2 shows __________.
A) a nitrogen-nitrogen triple bond
B) a nitrogen-nitrogen single bond
C) each nitrogen has one nonbonding electron pair
D) each nitrogen has two nonbonding electron pairs
E) each hydrogen has one nonbonding electron pair
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For resonance forms of a molecule or ion, __________.
A) one always corresponds to the observed structure
B) all the resonance structures are observed in various proportions
C) the observed structure is an average of the resonance forms
D) the same atoms need not be bonded to each other in all resonance forms
E) there cannot be more than two resonance structures for a given species
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the octet rule, magnesium most likely forms a __________ ion.
A) Mg2+
B) Mg2-
C) Mg6-
D) Mg6+
E) Mg-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
There are __________ unpaired electrons in the Lewis symbol for a (an) sodium ion.
A) 3
B) 2
C) 0
D) 4
E) 1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A valid Lewis structure of __________ cannot be drawn without violating the octet rule.
A) NI3
B) SO2
C) ICl5
D) SiF4
E) CO2
Correct Answer

verified
Correct Answer
verified
Short Answer
To produce maximum heat, an explosive compound should have __________ chemical bonds and decompose to molecule with __________ bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the bonds below, __________ is the least polar.
A) C, O
B) N, O
C) C, F
D) S, O
E) K, Br
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 141
Related Exams