Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following, all are valid units for a reaction rate except __________.
A) mol/L
B) M/s
C) mol/hr
D) g/s
E) mol/L-hr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A compound decomposes by a first-order process. If 17.0% of the compound decomposes in 60.0 minutes, the half-life of the compound is __________.
A) 141 minutes
B) 181 minutes
C) 198 minutes
D) 223 minutes
E) 325 minutes
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The kinetics of the reaction below were studied and it was determined that the reaction rate increased by a factor of 9 when the concentration of B was tripled. The reaction is __________ order in B. A + B → P
A) zero
B) first
C) second
D) third
E) one-half
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The order of the reaction in A is __________.
A) 1
B) 2
C) 3
D) 4
E) 0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction 2NO2 → 2NO + O2 Follows second-order kinetics. At 300°C, [NO2] drops from 0.0100 M to 0.00650 M in 100.0 s. The rate constant for the reaction is __________ M-1s-1.
A) 0.096
B) 0.65
C) 0.81
D) 1.2
E) 0.54
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The overall order of a reaction is 2. The units of the rate constant for the reaction are __________.
A) M/s
B) M-1s-1
C) 1/s
D) 1/M
E) s/M2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate constant for a second-order reaction is 0.13 M-1s-1. If the initial concentration of reactant is 0.26 mol/L, it takes __________ s for the concentration to decrease to 0.11 mol/L.
A) 0.017
B) 0.68
C) 9.1
D) 40.
E) 5.2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate of disappearance of HBr in the gas phase reaction 2HBr (g) → H2 (g) + Br2 (g) Is 0.301 Ms-1 at 150°C. The rate of appearance of Br2 is __________ Ms-1.
A) 1.66
B) 0.151
C) 0.0906
D) 0.602
E) 0.549
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g)
The dependence of the rate constant on temperature is studied and the graph below is prepared from the results. 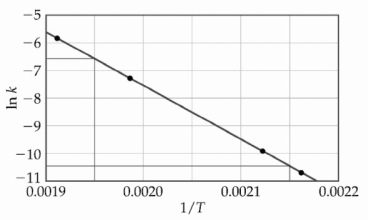 The energy of activation of this reaction is __________ kJ/mol.
The energy of activation of this reaction is __________ kJ/mol.
A) 160
B) 1.6 × 105
C) 4.4 × 10-7
D) 4.4 × 10-4
E) 1.9 × 104
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen fixation is a difficult process because __________.
A) there is so little nitrogen in the atmosphere
B) nitrogen exists in the atmosphere primarily as its oxides which are very unreactive
C) nitrogen is very unreactive, largely due to its triple bond
D) of the extreme toxicity of nitrogen
E) of the high polarity of nitrogen molecules preventing them from dissolving in biological fluids, such as those inside cells
Correct Answer

verified
Correct Answer
verified
Multiple Choice
__________ are used in automotive catalytic converters.
A) Heterogeneous catalysts
B) Homogeneous catalysts
C) Enzymes
D) Noble gases
E) Nonmetal oxides
Correct Answer

verified
Correct Answer
verified
True/False
Rates of reaction can be positive or negative.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The graph shown below depicts the relationship between concentration and time for the following chemical reaction. ![The graph shown below depicts the relationship between concentration and time for the following chemical reaction. The slope of this line is equal to __________. A) k B) -1/k C) ln[A]<sub>o</sub> D) -k E) 1/k](https://d2lvgg3v3hfg70.cloudfront.net/TB1822/11ea7ce9_fb1d_f542_b46a_4baffb1705df_TB1822_00.jpg) The slope of this line is equal to __________.
The slope of this line is equal to __________.
A) k
B) -1/k
C) ln[A]o
D) -k
E) 1/k
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the overall order of the reaction?
A) 4
B) 0
C) 1
D) 2
E) 3
Correct Answer

verified
Correct Answer
verified
True/False
Units of the rate constant of a reaction are independent of the overall reaction order.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At elevated temperatures, methylisonitrile (CH3NC) isomerizes to acetonitrile (CH3CN) : CH3NC (g) → CH3CN (g) At the start of the experiment, there are 0.200 mol of reactant (CH3NC) and 0 mol of product (CH3CN) in the reaction vessel. After 25 min of reaction, 0.108 mol of reactant (CH3NC) remain. The average rate of decomposition of methyl isonitrile, CH3NC, in this 25 min period is __________ mol/min.
A) 3.7 × 10-3
B) 0.092
C) 2.3
D) 4.3 × 10-3
E) 0.54
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction was found to be third order in A. Increasing the concentration of A by a factor of 3 will cause the reaction rate to __________.
A) remain constant
B) increase by a factor of 27
C) increase by a factor of 9
D) triple
E) decrease by a factor of the cube root of 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the Arrhenius equation, k = Ae-Ea/RT __________ is the frequency factor.
A) k
B) A
C) e
D) Ea
E) R
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many moles of B are present at 10 s?
A) 0.011
B) 0.220
C) 0.110
D) 0.014
E) 1.4 × 10-3
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 130
Related Exams