A) The reaction quotient must be satisfied for equilibrium to be achieved.
B) At equilibrium, the reaction quotient is undefined.
C) The reaction is at equilibrium when Q < Keq.
D) The reaction is at equilibrium when Q > Keq.
E) The reaction is at equilibrium when Q = Keq.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Keq for the equilibrium below is 0.112 at 700.0°C. SO2 (g) + 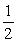 O2 (g)
O2 (g) 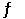 SO3 (g)
What is the value of Keq at this temperature for the following reaction?
2SO2 (g) + O2 (g)
SO3 (g)
What is the value of Keq at this temperature for the following reaction?
2SO2 (g) + O2 (g) 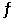 2SO3 (g)
2SO3 (g)
A) 0.224
B) 0.335
C) 0.0125
D) 0.0560
E) 0.112
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At equilibrium, __________.
A) all chemical reactions have ceased
B) the rates of the forward and reverse reactions are equal
C) the rate constants of the forward and reverse reactions are equal
D) the value of the equilibrium constant is 1
E) the limiting reagent has been consumed
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In what year was Fritz Haber awarded the Nobel Prize in chemistry for his development of a process for synthesizing ammonia directly from nitrogen and hydrogen?
A) 1954
B) 1933
C) 1918
D) 1900
E) 1912
Correct Answer

verified
Correct Answer
verified
Short Answer
Pure __________ and pure __________ are excluded from equilibrium-constant expressions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following expressions is the correct equilibrium-constant expression for the following reaction? CO2 (g) + 2H2 (g) 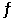 CH3OH (g)
CH3OH (g)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant for the gas phase reaction N2 (g) + O2 (g)  2NO (g)
Is Keq = 4.20 × 10-31 at 30°C. At equilibrium, __________.
2NO (g)
Is Keq = 4.20 × 10-31 at 30°C. At equilibrium, __________.
A) products predominate
B) reactants predominate
C) roughly equal amounts of products and reactants are present
D) only products are present
E) only reactants are present
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following expressions is the correct equilibrium-constant expression for the reaction below? 2SO2 (g) + O2 (g) ![Which of the following expressions is the correct equilibrium-constant expression for the reaction below? 2SO<sub>2</sub> (g) + O<sub>2</sub> (g) 2SO<sub>3</sub> (g) A) [SO<sub>3</sub>] / [SO<sub>2</sub>][O<sub>2</sub>] B) [SO<sub>2</sub>] / [SO<sub>3</sub>] C) [SO<sub>3</sub>]<sup>2</sup> / [SO<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>] D) [SO<sub>3</sub>]<sup>2</sup> / [SO<sub>2</sub>]<sup>2</sup>[O<sub>2</sub>]<sup>2</sup> E) [SO<sub>3</sub>] / [SO<sub>2</sub>][O<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB1822/11ea7ce9_fb24_3701_b46a_810d1452899c_TB1822_11.jpg) 2SO3 (g)
2SO3 (g)
A) [SO3] / [SO2][O2]
B) [SO2] / [SO3]
C) [SO3]2 / [SO2]2[O2]
D) [SO3]2 / [SO2]2[O2]2
E) [SO3] / [SO2][O2]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium constant for the gas phase reaction N2 (g) + 3H2 (g) 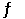 2NH3 (g)
Is Keq = 4.34 × 10-3 at 300°C. At equilibrium, __________.
2NH3 (g)
Is Keq = 4.34 × 10-3 at 300°C. At equilibrium, __________.
A) products predominate
B) reactants predominate
C) roughly equal amounts of products and reactants are present
D) only products are present
E) only reactants are present
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the coal-gasification process, carbon monoxide is converted to carbon dioxide via the following reaction: CO (g) + H2O (g) 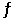 CO2 (g) + H2 (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.16 mol of CO remaining. Keq at the temperature of the experiment is __________.
CO2 (g) + H2 (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.16 mol of CO remaining. Keq at the temperature of the experiment is __________.
A) 5.5
B) 0.75
C) 0.93
D) 1.1
E) 1.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction at equilibrium. 2CO2 (g) 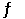 2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that the equilibrium partial pressure of CO (g) can be maximized by carrying out the reaction __________.
2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that the equilibrium partial pressure of CO (g) can be maximized by carrying out the reaction __________.
A) at high temperature and high pressure
B) at high temperature and low pressure
C) at low temperature and low pressure
D) at low temperature and high pressure
E) in the presence of solid carbon
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following will change the value of an equilibrium constant?
A) changing temperature
B) adding other substances that do not react with any of the species involved in the equilibrium
C) varying the initial concentrations of reactants
D) varying the initial concentrations of products
E) changing the volume of the reaction vessel
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Dinitrogentetraoxide partially decomposes according to the following equilibrium: N2O4 (g) 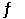 2NO2 (g)
A 1.00-L flask is charged with 0.0400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is __________.
2NO2 (g)
A 1.00-L flask is charged with 0.0400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is __________.
A) 2.2 × 10-4
B) 13
C) 0.22
D) 0.022
E) 0.87
Correct Answer

verified
Correct Answer
verified
True/False
At constant temperature, reducing the volume of a gaseous equilibrium mixture causes the reaction to shift in the direction that increases the number of moles of gas in the system.
Correct Answer

verified
Correct Answer
verified
Short Answer
If the reaction quotient Q for a reaction is greater than the value of the equilibrium constant K for that reaction at a given temperature, __________ must be converted to __________ for the system to reach equilibrium.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following chemical reaction: H2 (g) + I2 (g) 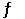 2HI (g)
At equilibrium in a particular experiment, the concentrations of H2, I2, and HI were 0.25 M, 0.035 M, and 0.55 M, respectively. The value of Keq for this reaction is __________.
2HI (g)
At equilibrium in a particular experiment, the concentrations of H2, I2, and HI were 0.25 M, 0.035 M, and 0.55 M, respectively. The value of Keq for this reaction is __________.
A) 23
B) 63
C) 0.0090
D) 5.1
E) 34
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What role did Karl Bosch play in development of the Haber-Bosch process?
A) He discovered the reaction conditions necessary for formation of ammonia.
B) He originally isolated ammonia from camel dung and found a method for purifying it.
C) Haber was working in his lab with his instructor at the time he worked out the process.
D) He developed the equipment necessary for industrial production of ammonia.
E) He was the German industrialist who financed the research done by Haber.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium expression for Kp for the reaction below is __________. 2O3 (g) 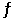 3O2 (g)
3O2 (g)
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
Exactly 3.5 moles if N2O4 is placed in an empty 2.0-L container and allowed to reach equilibrium described by the equation N2O4 (g)⇌ 2NO2 (g) If at equilibrium the N2O4 is 25% dissociated, what is the value of the equilibrium constant for the reaction?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The value of Keq for the equilibrium CO2 (g) + 2H2 (g) 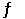 CH3OH (g)
Is 14.5 at 483 °C. What is the value of Keq for the equilibrium below?
1/2 CO2 + H2 (g)
CH3OH (g)
Is 14.5 at 483 °C. What is the value of Keq for the equilibrium below?
1/2 CO2 + H2 (g) 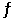 1/2 CH3OH (g)
1/2 CH3OH (g)
A) 7.30
B) 7.35
C) 0.136
D) 3.81
E) 6.90 × 10-2
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 92
Related Exams