A) H2
B) Cl2
C) CCl4
D) NaCl
Correct Answer

verified
Correct Answer
verified
Short Answer
The Lewis electron-dot structure of H2CO has ________ nonbonding electron pairs,________ bonding electron pairs,and a carbon-oxygen bond order of ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound ICl contains
A) ionic bonds.
B) nonpolar covalent bonds.
C) polar covalent bonds with partial negative charges on the Cl atoms.
D) polar covalent bonds with partial negative charges on the I atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The nitrogen-nitrogen bond in :N  N: has a bond order of
N: has a bond order of
A) 3
B) 1
C) 2
D) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following are allowed resonance forms of NCS-? I [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_7145_a2f7_2d1c6fb99396_TB4940_11.jpg) C -
C - ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_7146_a2f7_373eb22ef1c5_TB4940_11.jpg) :] - and [:
:] - and [: ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_9857_a2f7_079af94b3bc3_TB4940_11.jpg) = C =
= C = ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_9858_a2f7_712ed6cb2e0d_TB4940_11.jpg) :] -
II [: N
:] -
II [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_bf69_a2f7_1503286218fd_TB4940_11.jpg) C -
C - ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_bf6a_a2f7_3b7cb00b2527_TB4940_11.jpg) :] - and [: N
:] - and [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_bf6b_a2f7_63adc7e3eb54_TB4940_11.jpg) C =
C = ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_e67c_a2f7_51f22e6ff12d_TB4940_11.jpg) :] -
III [: N
:] -
III [: N ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c5_e67d_a2f7_63c442c8d6dc_TB4940_11.jpg) C -
C - ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c6_0d8e_a2f7_9f8c7794f49a_TB4940_11.jpg) :] - and [:
:] - and [: ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c6_0d8f_a2f7_dfed915d583d_TB4940_11.jpg) - C
- C ![Which of the following are allowed resonance forms of NCS<sup>-</sup>? I [: N C - :]<sup> -</sup> and [: = C = :] <sup>-</sup> II [: N C - :] <sup>-</sup> and<sup> </sup>[: N C = :] <sup>-</sup> III [: N C - :] <sup>-</sup> and [: - C N :] <sup>-</sup> A) only I B) only II C) only III D) I and III](https://d2lvgg3v3hfg70.cloudfront.net/TB4940/11ea7e2d_d0c6_34a0_a2f7_49ca047e2680_TB4940_11.jpg) N :] -
N :] -
A) only I
B) only II
C) only III
D) I and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compound A is a solid with a melting point of 85°C,and compound B is a gas at 75°C and one atmosphere pressure.Based on these data,one would expect
A) both compounds to be covalent.
B) compound A to be ionic and compound B to be covalent.
C) compound A to be covalent and compound B to be ionic.
D) both compounds to be ionic.
Correct Answer

verified
Correct Answer
verified
Essay
Among the compounds H3C-CH3,H2C=CH2,and HC  CH,the compound with the strongest carbon-carbon bond is ________,and the compound with the longest carbon-carbon bond is ________.
CH,the compound with the strongest carbon-carbon bond is ________,and the compound with the longest carbon-carbon bond is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the most acceptable electron-dot structure for carbonyl fluoride,COF2 the central atom is
A) C,which is singly-bonded to O.
B) C,which is doubly-bonded to O.
C) O,which is singly-bonded to C
D) O,which is doubly-bonded to C.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on formal charge considerations,the electron-dot structure of CO32- ion has
A) two resonance structures involving two single bonds and one double bond.
B) two resonance structures involving one single bond and two double bonds.
C) three resonance structures involving two single bonds and one double bond.
D) three resonance structures involving one single bond and two double bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When melting S8,________ forces must be overcome and S8 is expected to have a ________ melting point than MgS.
A) covalent bonding,higher
B) covalent bonding,lower
C) intermolecular,higher
D) intermolecular,lower
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs of electrons are on the I atom in IF5?
A) 0
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs in the correct electron dot structure of O3?
A) 2
B) 3
C) 4
D) 6
Correct Answer

verified
Correct Answer
verified
Short Answer
Based on formal charges,the P-O bond order in POCl3 is expected to be ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the approximate carbon-oxygen bond order in CO32-?
A) 2
B) 4/3
C) 5/3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange the following in order of increasing ionic character: Al2S3,MgS,Na2S,P4S3,S8.
A) MgS,Na2S,Al2S3,P4S3,S8
B) Na2S,MgS,Al2S3,P4S3,S8
C) S8,P4S3,Al2S3,MgS,Na2S
D) S8,P4S3,Al2S3,Na2S,MgS
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electronegativities for the elements vary from 0.7 for cesium to 4.0 for fluorine.The electronegativity for iodine is 2.5.Based entirely on the general guidelines for electronegativities and bond character,
A) binary compounds with iodine should all be polar covalent with a δ- on I.
B) binary compounds with iodine should all be polar covalent with a δ+ on I.
C) compounds with iodine may be ionic,polar covalent,or nonpolar covalent.
D) no binary compounds with iodine should be substantially ionic.
Correct Answer

verified
Correct Answer
verified
Short Answer
Of the bonds C-C,C-N,C-O,and C-F,the bond that is most polar is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs of electrons are on the N atom in NBr3?
A) 0
B) 1
C) 2
D) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assign formal charges to each atom in the resonance form for SOCl2 given below. 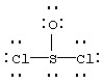
A) 0 for Cl,0 for S,and 0 for O
B) 0 for Cl,+1 for S,and -1 for O
C) -1 for Cl,+4 for S,and -2 for O
D) -1 for Cl,-2 for S,and -2 for O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two resonance forms for SOCl2 are given below. 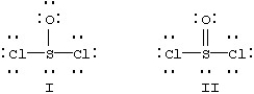 Which is favored by the octet rule and which by formal charge considerations?
Which is favored by the octet rule and which by formal charge considerations?
A) I is favored by the octet rule and by formal charge considerations.
B) I is favored by the octet rule and II by formal charge considerations.
C) II is favored by the octet rule and I by formal charge considerations.
D) II is favored by the octet rule and by formal charge considerations.
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 92
Related Exams