A) bent
B) tetrahedral
C) trigonal planar
D) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which drawing represents the molecular orbital containing the highest energy electrons in the F2 molecule in its ground state?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an electron is added to H2 it would go into a
A) σ molecular orbital and strengthen the H-H bond.
B) σ molecular orbital and weaken the H-H bond.
C) σ* molecular orbital and strengthen the H-H bond.
D) σ* molecular orbital and weaken the H-H bond.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry around the central atom in the following molecular model of PH3? 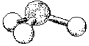
A) linear
B) bent
C) trigonal pyramidal
D) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hybridization of the nitrogen atom?
A) sp
B) sp2
C) sp3
D) sp4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Electrostatic potential maps use color to portray the calculated electron distribution in a molecule.Atoms that are electron poor and carry a δ+ charge are shown in blue.Atoms that are electron rich and carry a δ- charge are shown in red.Atoms with little or no charge are shown in green.The electrostatic potential map of H2O below should show 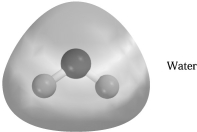
A) H blue and O red.
B) H blue and O green.
C) H green and O blue.
D) H red and O blue.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following should be nonlinear? 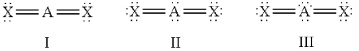
A) only I
B) only II
C) only III
D) II and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What orbital hybridization is expected for the central atom in a molecule with a trigonal planar geometry?
A) sp
B) sp2
C) sp3
D) sp4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule has a central atom that uses the set of hybrid orbitals shown below to form bonds with the non-central atoms? 
A) H2O
B) CO2
C) NO2-
D) ICl2-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which orbital hybridization is associated with a tetrahedral cloud arrangement?
A) sp
B) sp2
C) sp3
D) sp4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What geometric arrangement of charge clouds is expected for an atom that has five charge clouds?
A) tetrahedral
B) square planar
C) trigonal bipyramidal
D) octahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the geometry around the central atom in the following molecular model of H3O+? 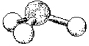
A) linear
B) bent
C) trigonal pyramidal
D) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which type of bond produces a charge cloud with a nodal plane containing the bond axis?
A) σ
B) π
C) σ and π
D) neither σ nor π
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the best description of the bonding in NO3-?
A) 3 N-O σ bonds and no N-O π bonds
B) 3 N-O σ bonds and 3 N-O π bonds
C) no N-O σ bonds and delocalized N-O π molecular orbitals extending over all four atoms
D) 3 N-O σ bonds and delocalized N-O π molecular orbitals extending over all four atoms
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The bonds in the polyatomic ion CO32- are classified as
A) ionic.
B) metallic.
C) nonpolar covalent.
D) polar covalent.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following MO diagram for Be2,Be2+,and Be2-.Based on this diagram, 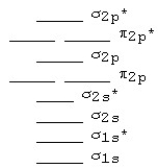
A) Be2+ is more stable than Be2,and Be2 is more stable than Be2-.
B) Be2- is more stable than Be2,and Be2 is more stable than Be2+.
C) Be2+ and Be2- are both more stable than Be2.
D) Be2 is more stable than either Be2+ or Be2-.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the bond angles in the following molecular model of SCl4? 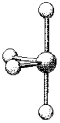
A) some less than 90° and one less than 180°
B) 90° and 180°
C) some less than 90° one less than 120° but greater than 90°,and one less than 180° but greater than 120°
D) 90°,120°,and 180°
Correct Answer

verified
Correct Answer
verified
Short Answer
Of NH4+ and NH4- the one with the smaller bond angles is ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The dipole moment of ClF is 0.88 D,and its bond length is 163 pm.What is the percent ionic character of the Cl-F bond?
A) 0.54%
B) 7.8%
C) 11%
D) 25%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Arrange in order from the smallest to the largest bond angle: CCl3+,NF3,NH4+,XeBr4.
A) CCl3+,NF3,NH4+,XeBr4
B) NF3,NH4+,XeBr4,CCl3+
C) XeBr4,NH4+,NF3,CCl3+
D) XeBr4,NF3,NH4+,CCl3+
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 205
Related Exams