A) -580 kJ
B) -35 kJ
C) +35 kJ
D) 580 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the freezing of liquid hexane,C6H,at a given temperature and pressure,
A) ΔH is negative and ΔS is negative.
B) ΔH is negative and ΔS is positive.
C) ΔH is positive and ΔS is negative.
D) ΔH is positive and ΔS is positive.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Imagine a reaction that results in a change in both volume and temperature,as shown in the diagram below.What is the sign of the work being done and the sign of the enthalpy change involved in this reaction? 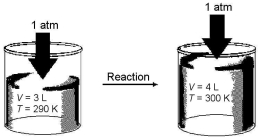
A) w = + and ΔH = +
B) w = + and ΔH = -
C) w = - and ΔH = +
D) w = - and ΔH = -
Correct Answer

verified
Correct Answer
verified
Short Answer
A property whose value depends only on the present condition of the system and not how the system arrived at that condition is called a ________ function.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Acetylene torches utilize the following reaction: 2 C2H2(g) + 5 O2(g) → 4 CO2(g) + 2 H2O(g)
Use the given standard enthalpies of formation to calculate ΔH° for this reaction. 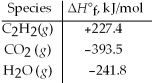
A) 1603kJ
B) -1256.2 kJ
C) 2512.4 kJ
D) -2512.4 kJ
Correct Answer

verified
Correct Answer
verified
Short Answer
The sum of the potential and kinetic energies for every molecule or ion in a system is the ________ energy of the system and is given the symbol ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Coal gasification can be represented by the equation: 2 C(s) + 2 H2O(g) → CH4(g) + CO2(g) ΔH = ? Use the following information to find ΔH for the reaction above. CO(g) + H2(g) → C(s) + H2O(g) ΔH = -131 kJ CO(g) + H2O(g) → CO2(g) + H2(g) ΔH = -41 kJ CO(g) + 3 H2(g) → CH4(g) + H2O(g) ΔH = -206 kJ
A) 15 kJ
B) 116 kJ
C) -116 kJ
D) -372 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant pressure for which of the reactions shown below should ΔH° be greater than ΔE°? I.2 SO2(g) + O2(g) → 2 SO3(g) II.C9H20(g) + 14 O2(g) → 9 CO2(g) + 10 H2O(l) III.H2(g) + F2(g) → 2 HF(g) IV.N2O4(g) → 2 NO2(g)
A) I
B) III
C) IV
D) II and IV
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a process at constant pressure,
A) ΔE = w and q = 0.
B) ΔE = q and w = 0.
C) ΔE = ΔH.
D) ΔH = q.
Correct Answer

verified
Correct Answer
verified
Short Answer
The law of conservation of energy is also known as the ________ law of thermodynamics.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is not a spontaneous process?
A) combustion of gasoline to produce carbon dioxide and water
B) diffusion of perfume in a room
C) dissolution of powdered punch in water
D) freezing of water at 3°C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a process at constant volume,
A) q = 0,w = 0,and ΔE = 0.
B) w = 0 and ΔE = q.
C) w = 0 and ΔH = q.
D) w = 0 and ΔE = ΔH.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At constant pressure,the combustion of 30.00 g of C2H6(g) releases 1560 kJ of heat.What is ΔH for the reaction given below? 2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(l)
A) -43.2 kJ
B) -779 kJ
C) -1560 kJ
D) -3120 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2CH4 (g) + 3 Cl2 (g) → 2 CHCl3 (l) + 3 H2 (g) ,ΔH° = -118.6 kJ. ΔH°f = -134.1 kJ/mol for CHCl3 (l) .Find ΔH°f for CH4 (g) .
A) -193.4 kJ/mol
B) -74.8 kJ/mol
C) 74.8 kJ/mol
D) 193.4 kJ/mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the total quantity of heat required to convert 50.0 g of liquid CCl4(l) from 25.0°C to gaseous CCl4 at 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 0.857 J/(g ∙ °C) ,its heat of fusion is 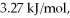 and its heat of vaporization is
and its heat of vaporization is 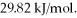
A) 2.22 kJ
B) 3.28 kJ
C) 11.91 kJ
D) 12.98 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas absorbs 0.0 J of heat and then performs 13.1 J of work.The change in internal energy of the gas is
A) 26.2 J
B) 13.1 J
C) -26.2 J
D) -13.1 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For most chemical reactions
A) ΔH is much larger than ΔE.
B) ΔE is much larger than ΔH.
C) ΔH is equal to ΔE.
D) the difference between ΔH and ΔE is very small.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When 2.500 mol of CH4(g) reacts with excess Cl2(g) at constant pressure according to the chemical equation shown below,1770.kJ of heat are released.Calculate the value of ΔH for this reaction,as written. 2 CH4(g) + 3 Cl2(g) → 2 CHCl3(l) + 3 H2(g) ΔH = ?
A) -1416 kJ
B) -708.0 kJ
C) +708.0 kJ
D) +1416 kJ
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For a particular process that is carried out at constant pressure,q = 140 kJ and w = -30 kJ.Therefore,
A) ΔE = 110 kJ and ΔH = 140 kJ.
B) ΔE = 140 kJ and ΔH = 110 kJ.
C) ΔE = 140 kJ and ΔH = 170 kJ.
D) ΔE = 170 kJ and ΔH = 140 kJ.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is expected when the reaction shown below takes place in a thermally-insulated container outfitted with a movable piston at a constant atmospheric pressure of 1 atm? 2 C2H6(g) + 7 O2(g) → 4 CO2(g) + 6 H2O(g)
A) Volume will decrease and work will be done by the system.
B) Volume will decrease and work will be done on the system.
C) Volume will increase and work will be done by the system.
D) Volume will increase and work will be done on the system.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 170
Related Exams