A) 5.26 × 1021 molecules
B) 5.54 × 1021 molecules
C) 1.59 × 1022 molecules
D) 1.67 × 1022 molecules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have two samples of the same gas in the same size container,with the same pressure.The gas in the first container has a Kelvin temperature four times that of the gas in the other container.The ratio of the number of moles of gas in the first container compared to that in the second is
A) 1:1.
B) 1:2.
C) 1:4.
D) 4:1.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is equivalent to 1 atm pressure?
A) 1.101325 bar
B) 1.00 bar
C) 20 psi
D) 740 mm Hg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the diagram below,nitrogen molecules are represented by unshaded spheres,oxygen molecules by gray spheres,and chlorine molecules by black spheres.  -If the total pressure in the container is 100.mm Hg,what is the partial pressure of chlorine?
-If the total pressure in the container is 100.mm Hg,what is the partial pressure of chlorine?
A) 10.0 mm Hg
B) 20.0 mm Hg
C) 30.0 mm Hg
D) 50.0 mm Hg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some assumptions from the kinetic molecular theory are listed below.Which one is most frequently cited to explain compressibility of a gas?
A) The average kinetic energy of gas particles is proportional to the Kelvin temperature.
B) Collisions of gas particles are elastic and total kinetic energy of the gas is constant.
C) A gas consist of tiny particles moving in random straight line motion.
D) The volume of the particles is negligible compared to the volume of the gas.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If CO2 and NH3 are allowed to effuse through a porous membrane under identical conditions,the rate of effusion for NH3 will be ________ times that of CO2.
A) 0.39
B) 0.62
C) 1.6
D) 2.6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
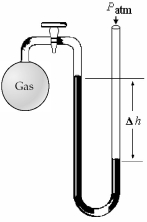 -What is the pressure (in mm Hg) of the gas inside the above apparatus if the outside pressure,Patm,is 740 mm Hg and the difference in mercury levels,Δh,is 30 mm Hg?
-What is the pressure (in mm Hg) of the gas inside the above apparatus if the outside pressure,Patm,is 740 mm Hg and the difference in mercury levels,Δh,is 30 mm Hg?
A) 30 mm Hg
B) 710 mm Hg
C) 740 mm Hg
D) 770 mm Hg
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If NO2 and NH3 are allowed to effuse through a porous membrane under identical conditions,the rate of effusion for NH3 will be ________ times that of CO2.
A) 0.37
B) 0.61
C) 1.6
D) 2.7
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Assume that you have a sample of gas in a cylinder with a moveable piston,as shown in diagram (1) .The initial pressure,number of moles,and temperature of the gas are noted on the diagram.Which diagram (2) -(4) most closely represents the result of doubling the pressure and number of moles of gas while keeping the temperature constant? 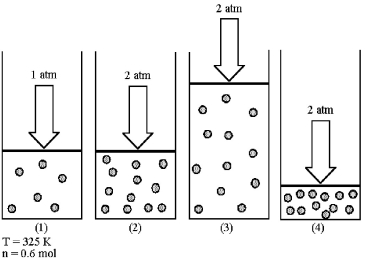
A) diagram (2)
B) diagram (3)
C) diagram (4)
Correct Answer

verified
Correct Answer
verified
Short Answer
The region of the atmosphere that is closest to the earth's surface is the ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A glass tube has one end in a dish of mercury and the other end closed by a stopcock.The distance from the surface of the mercury to the bottom of the stopcock is 800 mm,as indicated by the meter stick shown in the drawing below.The apparatus is at 20°C,and the mercury level in the tube is the same as that in the dish. 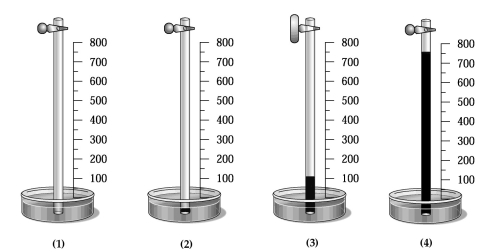 -Which drawing best shows the approximate level of the mercury in the tube when a vacuum pump is connected to the top of the tube,the stopcock opened,the tube is evacuated,the stopcock is closed,and the pump is removed?
-Which drawing best shows the approximate level of the mercury in the tube when a vacuum pump is connected to the top of the tube,the stopcock opened,the tube is evacuated,the stopcock is closed,and the pump is removed?
A) drawing (1)
B) drawing (2)
C) drawing (3)
D) drawing (4)
Correct Answer

verified
Correct Answer
verified
Short Answer
According to the kinetic molecular theory of gases,at 290 K the average kinetic energy of O2 is ________ the average kinetic energy of NO2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many liters of oxygen are needed to exactly react with 55.6 g of methane at STP? CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(l)
A) 19.5 L
B) 39.0 L
C) 156 L
D) 77.8 L
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the Earth's ozone (O3) layer has a total volume of 1.00 × 1020 km3,a partial pressure of 1.6 × 10-9 atm,and an average temperature of 230 K,how many ozone molecules are in the Earth's ozone layer?
A) 2.3 × 1035 molecules
B) 5.1 × 1035 molecules
C) 2.3 × 1045 molecules
D) 5.1 × 1045 molecules
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas bottle contains 0.800 mol of gas at 730 mm Hg pressure.If the final pressure is 1.15 atm,how many moles of gas were added to the bottle?
A) 0.0012 mol
B) 0.158 mol
C) 0.958 mol
D) 0.0668 mol
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A gas occupies 22.4 L at STP and 9.85 L at 100.°C and 2.00 atm pressure.How many moles of gas did the system gain or lose?
A) 1.36 moles gained
B) 1.64 moles gained
C) 0.644 moles lost
D) 0.356 moles lost
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following gases has the lowest average speed at 25°C?
A) C2H6
B) H2Se
C) PH3
D) F2
Correct Answer

verified
Correct Answer
verified
Short Answer
One mole of gas at 25°C has a ________ volume than one mole of gas at standard temperature.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A basketball is inflated to a pressure of 1.80 atm in a 23.0°C garage.What is the pressure of the basketball outside where the temperature is -2.00°C?
A) 1.65 atm
B) 1.70 atm
C) 1.90 atm
D) 1.97 atm
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Three identical flasks contain three different gases at standard temperature and pressure.Flask A contains 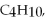 flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
flask B contains SO2,and flask C contains He. Which flask contains the largest number of molecules?
A) flask A
B) flask B
C) flask C
D) All contain same number of molecules.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 188
Related Exams