A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of 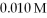 NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
A) 4.9 × 10-11 M
B) 4.9 × 10-10 M
C) 4.9 × 10-9 M
D) 7.0 × 10-6 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) . 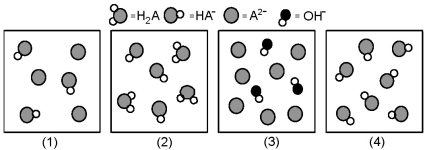 -Which picture represents the system halfway to the first equivalence point?
-Which picture represents the system halfway to the first equivalence point?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Short Answer
A buffer prepared by mixing equal moles of an acid having Ka = 4.5 × 10-4 and a salt of its conjugate base has a pH = ________.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a solution prepared by mixing 25.00 mL of 0.10 M CH3CO2H with 25.00 mL of 0.050 M CH3CO2Na? Assume that the volume of the solutions are additive and that Ka = 1.8 × 10-5 for CH3CO2H.
A) 2.87
B) 4.44
C) 4.74
D) 5.05
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these neutralization reactions has a pH < 7 when equal molar amounts of acid and base are mixed?
A) CH3CO2H(aq) + NaOH(aq) ⇌ H2O(l) + NaCH3CO2(aq)
B) HCl(aq) + C5H5N(aq) ⇌ C5H5NHCl(aq)
C) HBr(aq) + KOH(aq) ⇌ H2O(l) + KBr(aq)
D) H2SO4(aq) + 2 NaOH(aq) ⇌2 H2O(l) + Na2SO4(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH at the equivalence point of a weak base-strong acid titration if 20.00 mL of NaOCl requires 28.30 mL of 0.20 M HCl? Ka = 3.0 × 10-8 for HOCl.
A) 0.70
B) 3.39
C) 3.76
D) 4.23
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following plot shows a titration curve for the titration of 1.00 L of 1.00 M diprotic acid H2A with NaOH. 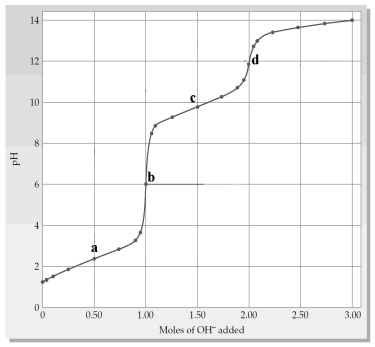 -Which point a-d represents the second equivalence point?
-Which point a-d represents the second equivalence point?
A) point a
B) point b
C) point c
D) point d
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions that contain a weak acid HA (pKa = 5.0) and its potassium salt KA.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity. ) 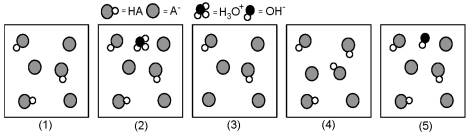 -Which picture represents the equilibrium state of the solution after addition of one OH- ion to the solution shown in picture (1) ?
-Which picture represents the equilibrium state of the solution after addition of one OH- ion to the solution shown in picture (1) ?
A) (2)
B) (3)
C) (4)
D) (5)
Correct Answer

verified
Correct Answer
verified
Short Answer
Identify the following solutions as acidic,basic,or inert. NaNO2 _______ NaNO3 ________ C5H5NHClO4 ________
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions of CaCO3,which may also contain ions other than Ca2+ and CO32- which are not shown.Gray spheres represent Ca2+ ions and unshaded spheres represent CO32- ions. 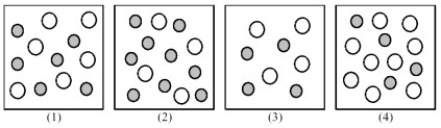 -If solution (1) is a saturated solution of CaCO3,which of solutions (1) -(4) represents the solution after a small amount of K2CO3 is added and equilibrium is restored?
-If solution (1) is a saturated solution of CaCO3,which of solutions (1) -(4) represents the solution after a small amount of K2CO3 is added and equilibrium is restored?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Short Answer
The neutralization constant Kn for the neutralization of penicillin V (C16H18N2O5S)and erythromycin (C37H67NO13)is 1.3 × 106.The acid dissociation constant Ka for penicillin V is 2.0 × 10-3.What is the base dissociation constant Kb for erythromycin?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following titrations result in an acidic solution at the equivalence point?
A) CH3COOH titrated with LiOH
B) NaF titrated with LiOH
C) HBr titrated with KOH
D) C5H5N titrated with HCl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a solution made by mixing 20.00 mL of 0.100 M HCl with 40.00 mL of 0.100 M KOH? Assume that the volumes of the solutions are additive.
A) 0.48
B) 1.48
C) 12.52
D) 13.52
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following pictures represent solutions at various stages in the titration of a weak diprotic acid H2A with aqueous KOH.Unshaded spheres represent H atoms,black spheres represent oxygen atoms,and shaded spheres represent A2- ions.(K+,H3O+ initially present,OH- initially present and solvent water molecules have been omitted for clarity) .  -Which picture represents the system halfway between the first and second equivalence points?
-Which picture represents the system halfway between the first and second equivalence points?
A) (1)
B) (2)
C) (3)
D) (4)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is not a correct expression for the weak acid HA?
A) Ka = [H3O+][A-]/[HA]
B) pKa = pH - log{[A-]/[HA]}
C) pKa = logKa
D) pKa = 14 - pKb
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The dissociation equilibrium constants for the protonated form of alanine (a diprotic amino acid,H2X+) are Ka1 = 4.6 × 10-3 and Ka2 = 2.0 × 10-10.What is the pH of 50.00 mL of a 0.0500 M solution of alanine after 25.00 mL of 0.100 M NaOH has been added?
A) 2.34
B) 4.85
C) 5.59
D) 6.72
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the hydronium ion concentration in a solution prepared by mixing 50.00 mL of 0.10 M HCN with 50.00 mL of 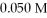 NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
NaCN? Assume that the volumes of the solutions are additive and that Ka = 4.9 × 10-10 for HCN.
A) 2.4 × 10-10 M
B) 4.9 × 10-10 M
C) 9.8 × 10-10 M
D) 7.0 × 10-6 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molar solubility of AgCl in 0.10 M NaCN if the colorless complex ion Ag(CN) 2- forms? Ksp for AgCl is 1.8 × 10-10 and Kf for Ag(CN) 2- is 1.0 × 1021.
A) 0.050 M
B) 0.10 M
C) 0.20 M
D) 0.40 M
Correct Answer

verified
Correct Answer
verified
Short Answer
The artist's pigment cadmium yellow,CdS,has a water solubility of 0.13 g/L.The solubility product of CdS,Ksp = ________.
Correct Answer

verified
Correct Answer
verified
Showing 181 - 200 of 201
Related Exams