A) CaSO4
B) NH4Cl
C) Mg(OH) 2
D) H2
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A formula unit of the ionic compound copper(II) carbonate consists of ________ copper(II) ions and ________ carbonate ions.
A) one; one
B) one; two
C) two; one
D) two; two
E) some other combination of ions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the most likely charge on an ion formed by an element with a valence electron configuration of ns2np4?
A) 2-
B) 1-
C) 2+
D) 4+
E) 6+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following formulas in incorrect for a cobalt(III) compound?
A) CoCO3
B) CoCl3
C) Co2O3
D) CoPO4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The name of Cl- is
A) chlorine ion.
B) chloride ion.
C) chlorate ion.
D) chlorite ion.
E) diatomic chlorine.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What fourth period element is represented by the dot structure shown? 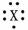
A) K
B) Ca
C) Mn
D) Br
E) Co
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The main group element E reacts with chlorine to form an ionic compound with the formula ECl2.The element E is a member of what group in the Periodic Table?
A) 1A
B) 2A
C) 6A
D) 7A
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the most likely charge on an ion formed by an element with a valence electron configuration of ns2np5?
A) 5-
B) 1-
C) 1+
D) 2+
E) 5+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the formula of the sulfite ion?
A) SO3 2-
B) SO4 2-
C) HSO4 2-
D) S 2-
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula PO4 3- means that this ion is composed of
A) one atom of phosphorus,one atom of oxygen,and three extra electrons.
B) four atoms of phosphorus,four atoms or oxygen,and three extra electrons.
C) one atom of phosphorus,four atoms of oxygen,and three electrons have been lost.
D) one atom of phosphorus,four atoms of oxygen,and three extra electrons.
E) four atoms of phosphorus,four atoms of oxygen,and three electrons have been lost.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element G could react with sulfur to form an ionic compound with formula GS2,the charge on the ion formed by G would be ________.
A) 4-
B) 2-
C) 1+
D) 2+
E) 4+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is the correct formula for the ionic compound containing iron(III) ions and oxide ions?
A) FeO
B) FeO2
C) Fe2O2
D) Fe2O3
E) Fe3O2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for ammonium hydroxide is ________.
A) OHNH4
B) NH4NO3
C) NH4O
D) NH4OH
E) Al(OH) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the name of AlCl3?
A) aluminum chloride
B) aluminum(III) chloride
C) aluminum carbide
D) aluminum trichloride
E) aluminum tricarbide
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula (NH4) 3PO4 indicates that one formula unit of this compound is composed of
A) three atoms of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,four atoms of oxygen,and six extra electrons.
B) three atoms of nitrogen,twelve atoms of hydrogen,four atoms of phosphorus,and four atoms of oxygen.
C) one atom of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
D) three atoms of nitrogen,seven atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
E) three atoms of nitrogen,twelve atoms of hydrogen,one atom of phosphorus,and four atoms of oxygen.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 75 of 75
Related Exams