A) endothermic;positive
B) exothermic;positive
C) endothermic;negative
D) exothermic;negative
E) exothermic;neither positive nor negative
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction is said to be ________ if the bonds formed during the reaction are stronger than the bonds broken.
A) exothermic
B) endothermic
C) exergonic
D) endergonic
E) spontaneous
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct figure to match the description. -A slow reaction with a large positive free energy change
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In order to determine if a reaction is at equilibrium which of the following must be true?
A) The concentrations of the reactants and products must be equal.
B) The concentration of the products must be greater than the reactants.
C) The forward and reverse reactions have stopped.
D) The rate at which the forward and reverse reactions are proceeding are equal.
E) All of the above are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct figure to match the description. -A slow reaction with a large negative free energy change
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Select the correct figure to match the description. -A fast reaction with a large negative free energy change
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When a reaction system is at equilibrium
A) there is no more chemistry happening.
B) the amounts of reactants and products are exactly equal.
C) the reaction rate in the forward direction is at a maximum.
D) the reaction rate in the reverse direction is at a minimum.
E) the rates of the reaction in the forward and reverse directions are exactly equal.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction shown: N2 + O2 → 2 NO ΔH = 43.2 kcal When 50.0 g of N2 react,________ kcal will be ________.
A) 43.2;produced
B) 77.1;consumed
C) 77.1;produced
D) 2160;consumed
E) 2160;produced
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the reaction A + B → AB,which of the following will not increase the rate?
A) adding A
B) adding B
C) increasing the temperature
D) decreasing the temperature
E) adding a catalyst
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction shown: 304.0 kcal + 4 PCl3(l) → P4(s) + 6 Cl2(g) When 50.00 g of PCl3 react,________ kcal will be ________.
A) 304.0;produced
B) 27.67;produced
C) 304.0;consumed
D) 110.7;consumed
E) 27.67;consumed
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
Which statement best describes the way a catalyst works?
A) It decreases the value of ΔH.
B) It increases the value of ΔH.
C) It decreases the value of Eact.
D) It increases the value of Eact.
E) It increases the value of ΔG.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match the following. -A process or reaction which consumes heat
A) exothermic
B) activation energy
C) chemical equilibrium
D) endergonic
E) exergonic
F) endothermic
Correct Answer

verified
Correct Answer
verified
Multiple Choice
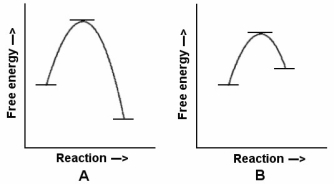 -In the reaction energy diagrams shown,reaction B is ________,and it occurs ________ reaction B.
-In the reaction energy diagrams shown,reaction B is ________,and it occurs ________ reaction B.
A) endergonic;faster than
B) exergonic;faster than
C) endergonic;slower than
D) exergonic;slower than
E) exergonic;at the same rate as
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following contains potential energy?
A) person riding a bicycle
B) a book on a table
C) a bouncing ball
D) a kid jumping rope
E) dancing
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following will affect all reaction rates?
A) the temperature of the reactants
B) the concentrations of the reactants
C) the presence of a catalyst
D) All are correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A reaction that is spontaneous can be described as
A) proceeding in both the forward and reverse directions.
B) having the same rate in both the forward and reverse directions.
C) releasing heat to the surroundings.
D) proceeding without external influence once it has begun.
E) increasing in disorder.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Based on the reaction shown,which statement is true? P4(s) + 10 Cl2(g) → 4 PCl5(s) ΔH = -435.2 kcal
A) When 1 mol P4(s) reacts,435.2 kcal are released.
B) When 1 mol PCl5(s) is produced,435.2 kcal are released.
C) When 30.97 g P4(s) react,435.2 kcal are released.
D) When 123.88 g P4(s) react,435.2 kcal are consumed.
E) When 208.22 g PCl5(s) are produced,435.2 kcal are consumed.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the equilibrium constant for the reaction below if a tank was found to contain 0.106 M O2,0.00652 M SO3, and 0.00129 M SO2.
2 SO3(g)  2 SO2(g) + O2(g)
2 SO2(g) + O2(g)
A) 6.78 × 10-2
B) 1.34 × 10-2
C) 4.15 × 10-3
D) 4.35 × 10-2
E) 9.66 × 10-3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following can affect the activation energy of a reaction?
A) increase in concentration of products
B) increase in concentration of reactants
C) surface area of reactants
D) use of a catalyst
E) temperature of the reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Write the equilibrium expression for the following reaction:
4 HNO3(l) ![Write the equilibrium expression for the following reaction: 4 HNO<sub>3</sub>(l) 4 NO<sub>2</sub>(g) + 2 H<sub>2</sub>O(g) + O<sub>2</sub>(g) A) [NO<sub>2</sub>]<sup>4</sup> [H<sub>2</sub>O]<sup>2</sup> [O<sub>2</sub>]/[HNO<sub>3</sub>]<sup>4</sup> B) 1/[NO<sub>2</sub>]<sup>4</sup> [H<sub>2</sub>O]<sup>2</sup> [O<sub>2</sub>] C) [HNO<sub>3</sub>]<sup>4</sup><sup>/</sup>[NO<sub>2</sub>]<sup>4</sup> [H<sub>2</sub>O]<sup>2</sup> [O<sub>2</sub>] D) [NO<sub>2</sub>]<sup>4</sup> [H<sub>2</sub>O]<sup>2</sup> [O<sub>2</sub>] E) [NO<sub>2</sub>]<sup>4</sup> + [H<sub>2</sub>O]<sup>2</sup> + [O<sub>2</sub>]](https://d2lvgg3v3hfg70.cloudfront.net/TB4943/11ea7e31_c17c_a511_9bba_cbad7990e944_TB4943_11.jpg) 4 NO2(g) + 2 H2O(g) + O2(g)
4 NO2(g) + 2 H2O(g) + O2(g)
A) [NO2]4 [H2O]2 [O2]/[HNO3]4
B) 1/[NO2]4 [H2O]2 [O2]
C) [HNO3]4/[NO2]4 [H2O]2 [O2]
D) [NO2]4 [H2O]2 [O2]
E) [NO2]4 + [H2O]2 + [O2]
Correct Answer

verified
D
Correct Answer
verified
Showing 1 - 20 of 81
Related Exams