A) protons
B) neutrons
C) protons and neutrons
D) protons, neutrons, and electrons
E) subatomic particles
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In the symbol below,X = ________. 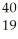 X
X
A) Zr
B) K
C) Sc
D) Br
E) not enough information to determine
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Aluminum forms an ion with a charge of ________.
A) 2+
B) 3-
C) 1+
D) 3+
E) 1-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An element in the upper right corner of the periodic table ________.
A) is either a metal or metalloid
B) is definitely a metal
C) is either a metalloid or a nonmetal
D) is definitely a nonmetal
E) is definitely a metalloid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formula for zinc phosphate is Zn3(PO4) 2.What is the formula for cadmium arsenate?
A) Cd4(AsO2) 3
B) Cd3(AsO4) 2
C) Cd3(AsO3) 4
D) Cd2(AsO4) 3
E) Cd2(AsO4) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species below is the nitride ion?
A) Na+
B) NO3-
C) NO2-
D) NH4+
E) N3-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An atom of 14C contains ________ electrons.
A) 14
B) 20
C) 8
D) 10
E) 6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element X has two naturally occurring isotopes.The masses (amu) and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 
A) 30.20
B) 33.20
C) 34.02
D) 35.22
E) 32.73
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many neutrons are there in one atom of 184W?
A) 74
B) 112
C) 258
D) 110
E) 184
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for Ni(CN) 2 is ________.
A) nickel (I) cyanide
B) nickel cyanate
C) nickel carbonate
D) nickel (II) cyanide
E) nickel (I) nitride
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is copper(I) chloride?
A) CuCl
B) CuCl2
C) Cu2Cl
D) Cu2Cl3
E) Cu3Cl2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The elements in groups 1A,6A,and 7A are called ________,respectively.
A) alkaline earth metals, halogens, and chalcogens
B) alkali metals, chalcogens, and halogens
C) alkali metals, halogens, and noble gases
D) alkaline earth metals, transition metals, and halogens
E) halogens, transition metals, and alkali metals
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The average atomic weight of copper,which has two naturally occurring isotopes,is 63.5.One of the isotopes has an atomic weight of 62.9 amu and constitutes 69.1% of the copper isotopes.The other isotope has an abundance of 30.9%.The atomic weight (amu) of the second isotope is ________ amu.
A) 63.2
B) 63.8
C) 64.1
D) 64.8
E) 28.1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for HClO is ________.
A) hydrochloric acid
B) perchloric acid
C) chloric acid
D) chlorous acid
E) hypochlorous acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following will occur as diatomic molecules in elemental form?
A) helium
B) argon
C) chlorine
D) phosphorous
E) sodium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many protons are there in one atom of 71Ga?
A) 40
B) 70
C) 71
D) 31
E) 13
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur forms an ion with a charge of ________.
A) 2+
B) 2-
C) 3+
D) 6-
E) 6+
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of the following,the smallest and lightest subatomic particle is the ________.
A) neutron
B) proton
C) electron
D) nucleus
E) alpha particle
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The element X has three naturally occurring isotopes.The isotopic masses (amu) and % abundances of the isotopes are given in the table below.The average atomic mass of the element is ________ amu. 
A) 161.75
B) 162.03
C) 162.35
D) 163.15
E) 33.33
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The correct name for Al2O3 is ________.
A) aluminum oxide
B) dialuminum oxide
C) dialuminum trioxide
D) aluminum hydroxide
E) aluminum trioxide
Correct Answer

verified
Correct Answer
verified
Showing 161 - 180 of 249
Related Exams