A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Small discrete packets of energy are known as
A) excited state energy.
B) ground state energy.
C) spectra of energy.
D) quanta of energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron configuration of an atom is 1s2 2s2 2p3.The number of unpaired electrons in this atom is
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which is not a noble gas?
A) Ra
B) Xe
C) Ar
D) Ne
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the maximum number of electrons that can occupy the 3d sublevel?
A) 2
B) 6
C) 10
D) 18
Correct Answer

verified
Correct Answer
verified
True/False
The 4s energy sublevel fills before the 3d energy sublevel.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On the periodic table,the "representative elements" are found in the
A) A Groups.
B) B Groups.
C) C Groups.
D) D Groups.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron configuration,[Ne] 3s2 3p4,is the ground state electron configuration of
A) sodium.
B) neon.
C) argon.
D) sulfur.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which principal energy level will contain electrons with the lowest energy?
A) First
B) Second
C) Third
D) Fourth
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The electron configuration of an atom is 1s2 2s2 2p2.The number of occupied orbitals in this atom is
A) 1
B) 2
C) 3
D) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ground state electron configuration for an atom of carbon is
A) 1s2 2s2
B) 1s2 2s2 2p2
C) 1s2 2s2 2p4
D) 1s2 2s4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which element is in the s-block of the periodic table?
A) Na
B) S
C) Pm
D) Mn
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Each vertical column on the periodic table is called a
A) series.
B) period.
C) cohort.
D) group.
Correct Answer

verified
Correct Answer
verified
True/False
The maximum number of valence electrons an atom can have is two.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following figure shows a(an) : 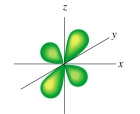
A) d orbital
B) p orbital
C) s orbital
Correct Answer

verified
Correct Answer
verified
Short Answer
Write orbital diagrams for the following elements in their ground states. A.Fluorine B.Oxygen C.Carbon
Correct Answer

verified
A.Fluorine 




1s 2s 2p...View Answer
Show Answer
Correct Answer
verified
1s 2s 2p...
View Answer
Multiple Choice
Which element is in the p-block of the periodic table?
A) Eu
B) Li
C) V
D) B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
On the periodic table,the "transition elements" fill their last electrons in the _____ sublevel.
A) s
B) p
C) d
D) f
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The line spectrum of different elements provides evidence for
A) electrons traveling in orbits around the nucleus
B) the quantization of the energy of the electrons
C) the ground-state of the atom
D) the existence of a nucleus
Correct Answer

verified
Correct Answer
verified
Showing 81 - 99 of 99
Related Exams