A) The electrons in the molecule are distributed evenly throughout the molecule.
B) The molecules are usually not attracted to one another very strongly.
C) Polar molecules have the weakest intermolecular interactions with ionic compounds.
D) The molecules have a high degree of symmetry.
E) None of these statements describe polar molecules.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would have the highest boiling point?
A) C6H14
B) C8H18
C) C10H22
D) C12H26
E) not enough information given
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is happening at the molecular level when a polar molecule like water interacts with a typical sodium ion?
A) The water molecule aligns such that the oxygen interacts with the sodium.
B) The water molecule aligns such that the hydrogens interact with the sodium.
C) The polarity of the water molecule is altered making the oxygen more positively charged.
D) The polarity of the water molecule is altered making the hydrogens more negatively charged.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How is the number of unpaired valence electrons in an atom related to the number of bonds that the atom can form?
A) There is no defined relationship between the number of unpaired valence electrons and number of bonds that the atom can form.
B) The number of unpaired valence electrons in an atom is one-half the number of bonds that the atom can form.
C) The number of unpaired valence electrons in an atom is twice the number of bonds that the atom can form.
D) The number of unpaired valence electrons in an atom is the same as the number of bonds that the atom can form.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would have the smallest number of induced dipole-induced dipole interactions?
A) C6H14
B) C8H18
C) C10H22
D) C12H26
E) not enough information given
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds has polar covalent bonds?
A) H2O
B) CsF2
C) S8
D) Ne
E) CH4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons does bromine (Br, atomic no. = 35) have?
A) 1
B) 7
C) 21
D) 28
E) 35
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the valence shell?
A) It is the outermost shell of electrons in an atom.
B) It is the shell of electrons in an atom that is the least reactive.
C) It is the last partially filled orbital in an atom.
D) It is the shell of electrons in element V (atomic no. = 23)
E) It is the same as the orbital configuration.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a neutral atom loses one electron, what is the electrical charge of the atom?
A) -1
B) +1
C) -2
D) +2
E) neutral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is it so easy for a magnesium atom to lose two electrons?
A) The nuclear charge of the magnesium atoms is relatively weak.
B) These two electrons are found relatively far from the nucleus.
C) These two electrons are well shielded from the nuclear charge.
D) There are lots of electron-electron repulsions that go on within the valence shell.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many valence electrons does gallium (Ga, atomic no. = 31) have?
A) 1
B) 6
C) 3
D) 31
E) 70
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements has two valence electrons?
A) Na
B) Mg
C) H
D) Ne
E) Li
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How are oxygen molecules attracted to water molecules?
A) The attraction between oxygen and water molecules is a classic example of dipole-dipole interaction.
B) The hydrogen bonding in water causes the attraction of the oxygen atoms in the O2 molecule to water.
C) As a water molecule is brought close to an oxygen molecule an induced dipole results in the O2 molecule causing the attraction.
D) The attraction of oxygen and water molecules for one another is part of the common atom effect. Since both molecules contain oxygen, there is a built-in attraction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the following diagram, describe what happens electronically between these two molecules. 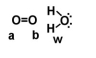
A) Oxygen A becomes slightly positively charged due to the protons on the water molecule.
B) Oxygen B becomes slightly positively charged due to the protons on the water molecule.
C) Oxygen A becomes slightly negatively charged due to the oxygen molecule.
D) Hydrogens on oxygen W becomes slightly positively charged due to the oxygen molecule.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following describes how a metal atoms behaves in a bulk metallic object?
A) The metal ion shares its outermost electrons freely with its neighbors.
B) The metal atoms have limited interaction with neighboring atoms.
C) The metal atom shares its electrons in a very directional manner.
D) The metal atom shares its electrons with only one other atom.
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Does an ionic bond have a dipole?
A) No, dipoles are only found in covalent compounds.
B) No, but the electrical charges are relatively strong.
C) Yes, the ionic bond is an example of a very strong dipole.
D) Yes, but for ionic compounds they are referred to as monopoles.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why are the melting temperatures of most ionic compounds far greater than the melting temperatures of most covalent compounds?
A) Ionic bonds are so much stronger than the molecular attractions between covalently bonded compounds.
B) Covalent bonds are not as strong as ionic bonds.
C) As a solid, salts have a very organized crystalline structure which takes a lot of energy to break apart.
D) Most covalent compounds have at least one weak bond in their structure that is easily broken when heat is added.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If carbonic acid (H2CO3) were to undergo ionization, what would one of the products be?
A) H-
B) CO2
C) H2O
D) CO3-1
E) CO3-2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which bond is most polar?
A) H-N
B) N-C
C) C-C
D) O-H
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following would be an ion with a double positive charge?
A) an Mg atom that gains two electrons
B) an Mg atom that gains one electron
C) an Mg atom that loses two electrons
D) an Mg atom that loses one electron
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 150
Related Exams