A) passed through the atomic nucleus
B) touched the atomic nucleus
C) were deflected by the atomic nucleus
D) were invisible to the atomic nucleus
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the particles of a cathode ray had a greater electric charge, the ray passing through a magnetic field would bend ________.
A) more because of a greater electromagnetic attraction
B) less because of greater electrical inertia
C) more because the particles would be traveling faster
D) by the same amount regardless of its charge
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If an element has 9 protons and 10 neutrons and 9 electrons, which expression correctly identifies the element?
A) ![]() F
F
B) ![]() F
F
C) ![]() K
K
D) ![]() K
K
E) ![]() K
K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which experiences a greater effective nuclear charge, an electron in the outer shell of neon (atomic number 10) or an electron in the outer shell of sodium (atomic number 11) ?
A) sodium because it contains more protons
B) neon because of less inner shell shielding
C) sodium because it contains a greater number of shells
D) neon because it is a smaller atom
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What do the electron configurations for all the group 18 noble gases have in common?
A) They have the same number of electrons.
B) They occupy the same number of shells.
C) Their outermost occupied shells are filled to capacity.
D) all of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following experiments helped to determine the charge of an electron and (indirectly) its mass?
A) J.J. Thompson's cathode ray deflection experiments
B) R. Millikan's oil drop experiments
C) E. Rutherford's gold foil experiments
D) Avogadro's number experiments
E) Franklin's kite experiments
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What do the components of a conceptual model have in common?
A) All components interact with each other.
B) All components are mutually independent of each other.
C) The components have nothing in common. This is what differentiates a conceptual model from a physical model.
D) Each component must correlate with a corresponding component of a physical model.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following atoms is the smallest?
A) Br
B) Ga
C) As
D) Ca
E) K
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rutherford assumed that the atomic nucleus was positively charged because ________.
A) electrons were already established to be negatively charged
B) the alpha particles that ricocheted off of the nucleus are also positively charged
C) he had a very optimistic outlook on life
D) Two of the above are reasonable answers
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Neon, Ne, (number 10) has a relatively large effective nuclear charge, yet it cannot attract any additional electrons because ________.
A) of the repulsion of neighboring electrons
B) the outermost shell has gotten too small
C) the outermost shell is too far away from the nucleus
D) its outermost shell is already filled to capacity
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What prompted early scientists to propose that the ray of the cathode ray tube was due to the cathode?
A) The ray was present regardless of gas, or even without a gas.
B) The ray was attracted to positively charge electric plates.
C) The ray was not seen from the positively charged anode.
D) The ray could be diverted by a magnetic field.
E) The ray would change colors depending on which gas was used inside the tube.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What color do you see when you close your eyes while in a dark room? Explain.
A) dark violet; Since no light is entering, you would tend to see the fringes of color from the ultra-violet region of the spectrum.
B) flashes of white; Although white is not really a color, you perceive all the frequencies of the spectrum as they flash by the retina of the eye.
C) black; Since no light is hitting the eye all you see is the absence of color, known as black.
D) all colors; The harder you close your eyes the more colors you will see.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mass number of an element is ________.
A) the sum of the protons and the neutrons
B) the sum of the electrons and the protons
C) the sum of the electrons and the neutrons
D) the sum of the isotopes
E) the number of protons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using the following generic atom description, choose the correct method for determining the number of neutrons. 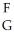 X
X
A) subtract G from F
B) subtract F from G
C) add F and G
D) divide F by G
E) look it up on the periodic table
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Evidence for the existence of neutrons did not come until many years after the discoveries of the electron and the proton. Give a possible explanation.
A) The neutron is nearly massless.
B) The neutron is only slightly more massive than the proton.
C) The discovery required the use of ultrafast computers.
D) The neutron lacks an electrical charge.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the above has the least energy?
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope lithium-7 has a mass of 7.0160 atomic mass units, and the isotope lithium-6 has a mass of 6.0151 atomic mass units. Given the information that 92.58 percent of all lithium atoms found in nature are lithium-7 and 7.42 percent are lithium-6, calculate the atomic mass of lithium, Li (atomic number 3) .
A) 7.0160 amu
B) 6.942 amu
C) 6.495 amu
D) 13.031 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following statement describes which subatomic particle best? It is located outside of the nucleus.
A) an electron
B) a proton
C) a neutron
D) A and B
E) B and C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following concepts underlies all the others: ionization energy, effective nuclear charge, atomic size.
A) ionization energy
B) effective nuclear charge
C) atomic size
D) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How might you distinguish a sodium-vapor street lamp from a mercury-vapor street lamp?
A) use binoculars to look for printing adjacent to the bulb
B) look at the street lamps through a spectroscope and match their spectral patterns to their respective atomic spectra
C) ask your local utility company to identify these lamps for you
D) The shadows cast from a sodium-vapor street lamp tend to be more diffuse around the edges.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 146
Related Exams