A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which molecule or compound below contains a polar covalent bond?
A) C2H4
B) ZnS
C) LiI
D) NCl3
E) AgCl
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Choose the bond below that is the weakest.
A) C≡O
B) N≡N
C) C-I
D) C=S
E) K-Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Give the number of valence electrons for SF4.
A) 28
B) 30
C) 32
D) 34
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the bond energies provided to estimate ΔrH° for the reaction below. XeF2 + 2F2 → XeF6 ΔrH° = ? Bond Bond Energy (kJ mol-1) Xe-F 147 F-F 159
A) -429 kJ mol-1
B) +159 kJ mol-1
C) -660 kJ mol-1
D) +176 kJ mol-1
E) -270 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the bond below that is least polar.
A) P-F
B) C-Br
C) C-F
D) C-I
E) C-Cl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the best Lewis structure for SO42-.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the compound or element with metallic bonding.
A) NaCl
B) Li
C) H2O
D) He
E) S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the bond below that is the weakest.
A) Na-Cl
B) I-I
C) C=N
D) Li-F
E) C=O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represent the Lewis structure for Ca2+?
A)
B)
C) ![]()
D) ![]()
E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following processes is exothermic?
A) Cl2(g) → 2Cl(g)
B) Br(g) + e- → Br- (g)
C) Li(s) → Li(g)
D) NaF(s) → Na+ (g) + F- (g)
E) None of the above processes is exothermic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The compound ClF contains
A) ionic bonds.
B) nonpolar covalent bonds.
C) polar covalent bonds with partial negative charges on the F atoms.
D) polar covalent bonds with partial negative charges on the Cl atoms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Using periodic trends,place the following bonds in order of increasing ionic character. Si-P Si-Cl Si-S
A) Si-P < Si-Cl < Si-S
B) Si-P < Si-S < Si-Cl
C) Si-S < Si-Cl < Si-P
D) Si-Cl < Si-P < Si-S
E) Si-Cl < Si-S < Si-P
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the number of valence electrons for XeI2.
A) 22
B) 20
C) 18
D) 24
Correct Answer

verified
Correct Answer
verified
Short Answer
The Lewis structure of BrO3- is as follows: 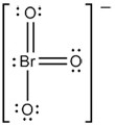 What is the formal charge on Br atom?
What is the formal charge on Br atom?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Place the following elements in order of increasing electronegativity. Sr N Na
A) Sr < Na < N
B) Na < N < Sr
C) Sr < N < Na
D) N < Sr < Na
E) N < Na < Sr
Correct Answer

verified
A
Correct Answer
verified
Essay
Draw the Lewis dot structure for Al3+.
Correct Answer

verified
The Al should have n...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Using Lewis structures and formal charge,which of the following ions is most stable? OCN- ONC- NOC-
A) OCN-
B) ONC-
C) NOC-
D) None of these ions is stable according to Lewis theory.
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Use the data given below to construct a Born-Haber cycle to determine the second ionization energy of Ca.
A) 1010 kJ mol-1
B) 1757 kJ mol-1
C) 1508 kJ mol-1
D) -3027 kJ mol-1
E) -1514 kJ mol-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the substance that conducts electricity.
A) NaCl dissolved in water
B) solid NaCl
C) water
D) solid sugar
E) sugar dissolved in water
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 136
Related Exams