A) 7.1 × 10-5 M
B) 4.2 × 10-10 M
C) 8.7 × 10-10 M
D) 6.5 × 10-5 M
E) 1.4 × 10-10 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the acid that is used to preserve soft drinks.
A) citric acid
B) hydrochloric acid
C) carbonic acid
D) hydrofluoric acid
E) phosphoric acid
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the pH of a 0.461 M C6H5CO2H M solution if the Ka of C6H5CO2H is 6.5 × 10-5.
A) 2.26
B) 4.52
C) 11.74
D) 9.48
E) 5.48
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the acid that is in car batteries.
A) H2SO4
B) HNO3
C) Na2CO3
D) CH3COOH
E) HCl
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Identify the substance that is NOT acidic.
A) soft drink
B) apple
C) household ammonia
D) lemon
E) plum
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which Br∅nsted-Lowry acid is NOT considered to be a strong acid in water?
A) HBr
B) HBr
C) HNO2
D) HNO3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the concentration of bicarbonate ion,HCO3-,in a 0.010 M H2CO3 solution that has the stepwise dissociation constants Ka1 = 4.3 × 10-7 and Ka2 = 5.6 × 10-11.
A) 6.6 × 10-5 M
B) 4.3 × 10-7 M
C) 4.3 × 10-9 M
D) 5.6 × 10-11 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of a 0.30 M pyridine solution that has Kb = 1.9 × 10-9? The equation for the dissociation of pyridine is: C5H5N(aq) + H2O(l) ⇌ C5H5NH+(aq) + OH-(aq)
A) 4.62
B) 8.72
C) 9.38
D) 10.38
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pH of pure water at 40.0°C if the Kw at this temperature is 2.92 × 10-14?
A) 6.767
B) 0.465
C) 7.000
D) 7.233
E) 8.446
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bases is the strongest? The base is followed by its Kb value.
A) (CH3CH2) 2NH, 8.6 × 10-4
B) CH3NH2, 4.4 × 10-4
C) C6H5NH2, 4.0 × 10-10
D) NH3, 1.76 × 10-5
E) C5H5N, 1.7 × 10-9
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon dioxide combines with rainwater to form
A) phosphoric acid.
B) carbonic acid.
C) nitric acid.
D) sulfuric acid.
E) nitrous acid.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Which of the following is an Arrhenius base?
A) CH3CO2H
B) RbOH
C) CH3OH
D) KBr
E) More than one of these compounds is an Arrhenius base.
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Determine the concentration of CO32- ions in a 0.18 M H2CO3 solution.Carbonic acid is a diprotic acid whose Ka1 = 4.3 × 10-7 and Ka2 = 5.6 × 10-11.
A) 2.8 × 10-4 M
B) 3.2 × 10-6 M
C) 5.6 × 10-11 M
D) 4.3 × 10-7 M
E) 6.9 × 10-8 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the pH of a 1.60 M KBrO solution.Ka for hypobromous acid,HBrO,is 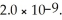
A) 2.55
B) 4.25
C) 9.75
D) 11.45
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following will form an acidic solution in water?
A) NH4Cl
B) LiF
C) NaI
D) LiNO3
E) None of the above solutions will be acidic.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Find the percent ionization of a 0.337 M HF solution.The Ka for HF is 3.5 × 10-4.
A) 1.1%
B) 1.2 × 10-2%
C) 3.2%
D) 3.5 × 10-2%
E) 4.7%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The stronger the acid,then which of the following is TRUE?
A) the stronger the conjugate acid
B) the stronger the conjugate base
C) the weaker the conjugate base
D) the weaker the conjugate acid
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Determine the [H3O⁺] in a 0.265 M HClO solution.The Ka of HClO is 2.9 × 10-8.
A) 1.1 × 10-10 M
B) 7.7 × 10-9 M
C) 1.3 × 10-6 M
D) 4.9 × 10-4 M
E) 8.8 × 10-5 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A solution with a hydrogen ion concentration of 3.25 × 10-6 M is ________ and has a hydroxide ion concentration of ________.
A) acidic, 3.08 × 10-8 M
B) acidic, 3.08 × 10-9 M
C) basic, 3.08 × 10-8 M
D) basic, 3.08 × 10-9 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following solutions would have the highest pH? Assume that they are all 0.10 M in acid at 25∘C.The acid is followed by its Ka value.
A) HF, 3.5 × 10-4
B) HCN, 4.9 × 10-10
C) HNO2, 4.6 × 10-4
D) HCHO2, 1.8 × 10-4
E) HClO2, 1.1 × 10-2
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 160
Related Exams