A) IO- is a catalyst.
B) I- is a catalyst.
C) The net reaction is 2H2O2 2H2O + O2.
D) The reaction is first-order with respect to [I-].
E) The reaction is first-order with respect to [H2O2].
Correct Answer

verified
Correct Answer
verified
Short Answer
Elementary steps in a reaction mechanism often include reaction ________.These (usually)short-lived species,which are at one point produced and then later consumed,do not appear in the overall chemical reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following units are consistent with the units of the reaction rate in a first order reaction?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the first-order decomposition of N2O5 at a high temperature,determine the rate constant if the N2O5 concentration decreases from 1.04 M to 0.62 M in 375 seconds.
A) 5.99 10-4 s-1
B) 1.59 10-3 s-1
C) 1.74 10-3 s-1
D) 1.38 10-3 s-1
E) 1.94 102 s-1
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction,A + 2B B2 + A,proceeds by the following mechanism: (A is a catalyst.) A + B AB (slow) AB + B B2 + A (fast) What is the rate law expression for this reaction?
A) Rate = k[A]
B) Rate = k[B]
C) Rate = k[A][B]
D) Rate = k[A][B]2
E) Rate = k[A]2[B]
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How are the exponents in a rate law determined?
A) They are equal to the inverse of the coefficients in the overall balanced chemical equation.
B) They are determined by experimentation.
C) They are equal to the coefficients in the overall balanced chemical equation.
D) They are equal to the reactant concentrations.
E) They are equal to the ln(2) divided by the rate constant.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the half-life of a first-order reaction if it takes 4.4 10-2 seconds for the concentration to decrease from 0.50 M to 0.20 M?
A) 2.5 10-2 s
B) 3.3 10-2 s
C) 1.6 s
D) 21 s
E) 27 s
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
A first-order chemical reaction is observed to have a rate constant of 34 min-1.What is the corresponding half-life for the reaction?
A) 1.2 s
B) 1.2 min
C) 49 min
D) 1.8 s
E) 48.6 s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the overall order of the reaction below
NO(g) + O3(g) NO2(g) + O2(g)
If it proceeds via the following rate expression? 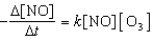
A) zero-order
B) first-order
C) second-order
D) third-order
E) fourth-order
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
A catalyst ____.
A) is used up in a chemical reaction
B) changes the potential energy change of the reaction
C) is always a solid
D) does not influence the reaction in any way
E) changes the activation energy of the reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon dioxide.
NO2(g) + CO(g) NO(g) + CO2(g)
A proposed mechanism for this reaction is
![Nitrogen dioxide reacts with carbon monoxide to produce nitrogen monoxide and carbon dioxide. NO<sub>2</sub>(g) + CO(g) \to NO(g) + CO<sub>2</sub>(g) A proposed mechanism for this reaction is What is a rate law that is consistent with the proposed mechanism? A) rate = k[NO<sub>2</sub>]<sup>2</sup>[CO] [NO]<sup>-1</sup> B) rate = k[NO<sub>2</sub>]<sup>2</sup>[CO] C) rate = k[NO<sub>2</sub>][CO] D) rate = k[NO<sub>3</sub>][CO] E) rate = k[NO<sub>2</sub>]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ec6bce_50b0_edc4_ac74_4345ebe789f1_TB4499_11.jpg) What is a rate law that is consistent with the proposed mechanism?
What is a rate law that is consistent with the proposed mechanism?
A) rate = k[NO2]2[CO] [NO]-1
B) rate = k[NO2]2[CO]
C) rate = k[NO2][CO]
D) rate = k[NO3][CO]
E) rate = k[NO2]2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In general,as temperature increases,the rate of a chemical reaction
A) decreases due to fewer collisions with proper molecular orientation.
B) increases for exothermic reactions,but decreases for endothermic reactions.
C) increases due to a greater number of effective collisions.
D) remains unchanged.
E) decreases due to an increase in the activation energy.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A possible mechanism for the gas phase reaction of NO and H2 is as follows:
![A possible mechanism for the gas phase reaction of NO and H<sub>2</sub> is as follows: Which of the following statements concerning this mechanism is not directly supported by the information provided? A) Step 1 is the rate determining step. B) N<sub>2</sub>O<sub>2</sub> is an intermediate. C) There is no catalyst in this reaction. D) The rate expression for step 1 is rate = k[NO]<sup>2</sup>. E) All steps are bimolecular reactions.](https://d2lvgg3v3hfg70.cloudfront.net/TB4499/11ec6bce_db57_0226_ac74_076589b58af3_TB4499_11.jpg) Which of the following statements concerning this mechanism is not directly supported by the information provided?
Which of the following statements concerning this mechanism is not directly supported by the information provided?
A) Step 1 is the rate determining step.
B) N2O2 is an intermediate.
C) There is no catalyst in this reaction.
D) The rate expression for step 1 is rate = k[NO]2.
E) All steps are bimolecular reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reactants A and B are mixed,and the reaction is timed until a color change occurs.The data are as follows:
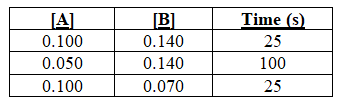 The order of the reaction with respect to reactant A is
The order of the reaction with respect to reactant A is
A) 2.
B) ![]() .
.
C) 1.
D) ![]() .
.
E) 0.
Correct Answer

verified
Correct Answer
verified
Short Answer
The ________ of an elementary step is defined as the number of reactant molecules that come together in the reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Given the initial rate data for the reaction A + B C,determine the rate expression for the reaction.
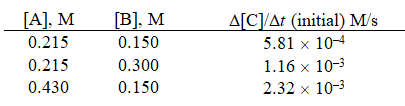
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
E
Correct Answer
verified
Multiple Choice
For the second-order reaction below,the initial concentration of reactant A is 0.24 M.If the rate constant for the reaction is 1.5 10-2 M-1s-1,what is the concentration of A after 265 seconds? 2A B + C rate = k[A]2
A) 0.12 M
B) 0.19 M
C) 0.95 M
D) 4.0 M
E) 5.2 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 2A + B C,the rate law is
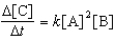 .
Which of the factor(s) will affect the value of the for this reaction?
1) decreasing the temperature
2) adding a catalyst
3) decreasing the concentration of reactant A
.
Which of the factor(s) will affect the value of the for this reaction?
1) decreasing the temperature
2) adding a catalyst
3) decreasing the concentration of reactant A
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2
E) 2 and 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The average rate of disappearance of ozone in the following reaction is found to be 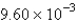 atm/s.
atm/s. 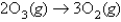 What is the rate of appearance of
What is the rate of appearance of 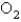 During this interval?
During this interval?
A) ![]() atm/s
atm/s
B) ![]() atm/s
atm/s
C) ![]() atm/s
atm/s
D) ![]() atm/s
atm/s
E) ![]() atm/s
atm/s
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The rate constant for a particular reaction is 0.0040 M.s-1.What is the overall order of this reaction?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 84
Related Exams