Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams shows a concerted endothermic reaction?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Draw curved arrows for the following mechanistic step. 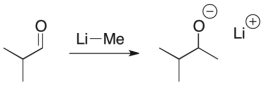
Correct Answer

verified
 _TB4454_00 6.10 Desc...
_TB4454_00 6.10 Desc...View Answer
Show Answer
Correct Answer
verified
View Answer
Short Answer
Does a reaction with a ∆H of 20 kJ/mol and a ∆S of 10 J/mol·K at 298 K favor reactants or products?
Correct Answer

verified
Correct Answer
verified
Short Answer
What is the energy of activation for the following reaction? 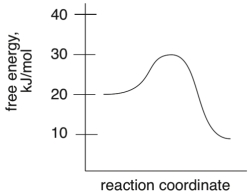
Correct Answer

verified
10 kJ/mol
Correct Answer
verified
Multiple Choice
Why is the entropy change negative for ring closures?
A) Closing a ring results in fewer molecules.
B) Closing a ring results in more molecules.
C) Closing a ring releases energy.
D) Closing a ring restricts the rotation around individual carbon-carbon bonds.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the sign of ∆G for an exothermic reaction with a decrease in entropy.
A) positive
B) negative
C) no change
D) cannot predict without additional information
Correct Answer

verified
Correct Answer
verified
Short Answer
Does a reaction with a Keq = 10 favor reactants or products?
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a homolytic bond cleavage, ____ are formed.
A) ions
B) radicals
C) only cations
D) only anions
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following indicates a reaction with a negative ∆G?
A) endergonic, spontaneous
B) endergonic, not spontaneous
C) exergonic, spontaneous
D) exergonic, not spontaneous
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Later in the course, we will compare the halogenation of differently substituted carbons, comparing reactions like the ones below. Which of the following reactions has a more exothermic heat of reaction (∆Ho) ? 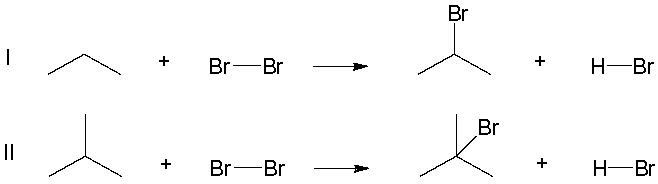
A) Reaction I has a more exothermic heat of reaction (∆Ho)
B) Reaction II has a more exothermic heat of reaction (∆Ho)
C) Both reactions have the same heat of reaction (∆Ho)
Correct Answer

verified
B
Correct Answer
verified
Short Answer
Given the following rate law, what is the order of the reaction with respect to sodium cyanide? 
Correct Answer

verified
second
Correct Answer
verified
Essay
Identify the nucleophilic centers in the following molecule. 
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction step, indicate which pattern of arrow pushing it represents. 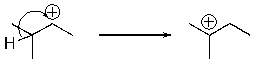
A) proton transfer
B) loss of leaving group
C) nucleophilic attack
D) rearrangement
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the arrow in the following equation. 
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
Using Table 6.1, estimate the enthalpy change of the following reaction under standard conditions. 
Correct Answer

verified
Correct Answer
verified
Essay
The following reaction has three mechanistic steps. Draw all curved arrows necessary to complete the mechanism. 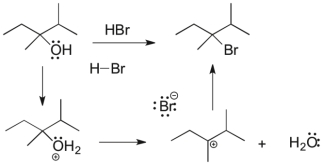
Correct Answer

verified
Correct Answer
verified
Short Answer
Predict the sign of ∆S for the following reaction. 
Correct Answer

verified
Correct Answer
verified
Essay
Identify the electrophilic site in the following molecule. 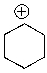
Correct Answer

verified
SHAPE \* M...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is a transition state?
A) An isolable intermediate in a reaction.
B) The starting materials of the reaction.
C) A local maximum on the energy diagram.
D) A low-energy point between the starting materials and the product.
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 96
Related Exams