Correct Answer

verified
 _TB4454_00 6.10 Desc...
_TB4454_00 6.10 Desc...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the effect of a catalyst on a reaction?
A) It increases the rate.
B) It decreases the entropy.
C) It changes the equilibrium.
D) It makes the products more stable.
Correct Answer

verified
Correct Answer
verified
Essay
Draw an energy diagram for an endothermic reaction with two steps.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of following reactions, which one(s) would you expect to have a negative ∆S? 
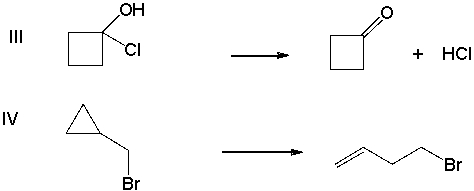
A) I
B) II
C) III
D) IV
E) I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following energy diagrams shows the reaction with the smallest energy of activation?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Short Answer
Based on the following energy diagram, is the reaction exothermic or endothermic? 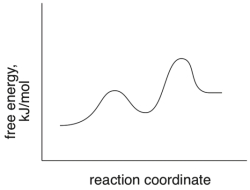
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the sign of ∆G for an exothermic reaction with an increase in entropy.
A) positive
B) negative
C) no change
D) cannot predict without additional information
Correct Answer

verified
Correct Answer
verified
Essay
Identify the nucleophilic site(s) in the following molecule. 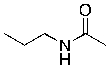
Correct Answer

verified
Correct Answer
verified
Short Answer
Based on the following energy diagram, is the reaction exothermic or endothermic? 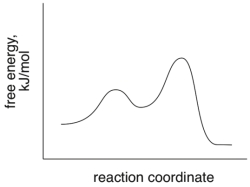
Correct Answer

verified
Correct Answer
verified
Short Answer
Does a reaction with a positive ∆G favor reactants or products?
Correct Answer

verified
Correct Answer
verified
Essay
Draw arrows for each step of the following reaction. 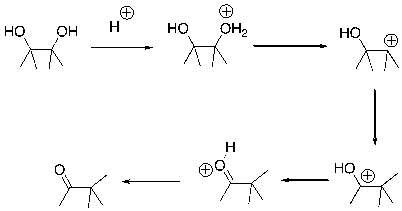
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the arrow in the following equation. 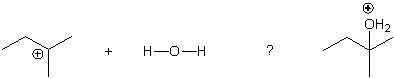
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following indicates a reaction with a positive ∆G?
A) endergonic, spontaneous
B) endergonic, not spontaneous
C) exergonic, spontaneous
D) exergonic, not spontaneous
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is wrong with the following mechanism? 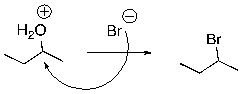
A) There is no leaving group, so there should be no arrows.
B) The arrow should be removing a proton from the H2O group.
C) An arrow is also needed to indicate the loss of the leaving group.
D) The arrow is backwards.
Correct Answer

verified
Correct Answer
verified
Essay
Draw an energy diagram for a concerted exothermic reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the sign of ∆S for the following reaction. SHAPE \* MERGEFORMAT 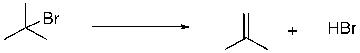

A) positive
B) negative
C) no change
Correct Answer

verified
Correct Answer
verified
Showing 81 - 96 of 96
Related Exams