A) CH3CH2CH2CH2CH2CH3
B) (CH3CH2) 2CHCH3
C) (CH3) 3CCH2CH3
D) (CH3) 2CHCH(CH3) 2
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is consistent with the following IR spectrum? 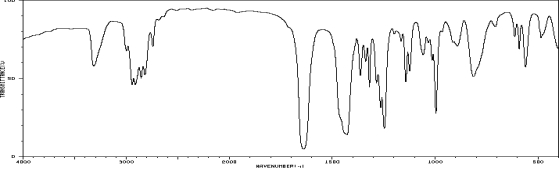 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds will show an absorption at 2250 cm-1? 
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Which of the following alkene groups will produce the stronger signal? Explain why. 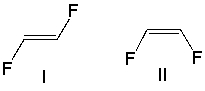
Correct Answer

verified
Compound II.
The C=C in compou...View Answer
Show Answer
Correct Answer
verified
The C=C in compou...
View Answer
Multiple Choice
Which of the following electromagnetic radiation has the highest energy?
A) UV
B) X-ray
C) IR
D) microwave
E) visible
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds will show two sharp absorptions, one at 3300 cm-1 and one at 2150 cm-1? 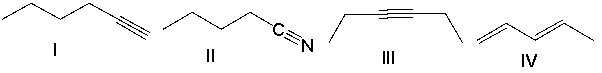
A) I
B) II
C) III
D) IV
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is consistent with the mass spectrum below? 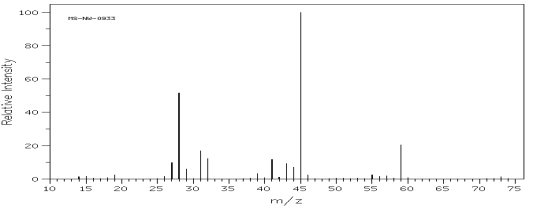 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology
A) CH3CH2CH(CH3) 2
B) CH3CHOHCH2CH3
C) CH3CH2OCH2CH3
D) CH3CH2NHCH2CH3
E) CH3CH2CH2CH3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds will have the lowest wavenumber for the carbonyl absorption? 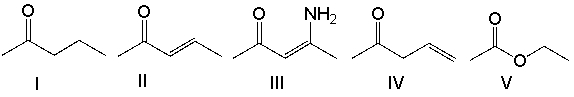
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
For the following pair of compounds the expected stretching absorption of the C=O bond is 1685 cm-1 and 1655 cm-1 respectively. Explain using both words and structural drawings. 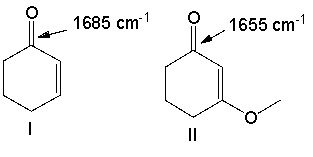
Correct Answer

verified
Both compounds I and II have conjugated ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following compounds will produce a prominent (M-15) peak in the mass spectrum?
A) 2-methylheptane
B) 1-heptanol
C) heptanamine
D) 1-chloroheptane
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds will produce a prominent (M-18) peak in the mass spectrum?
A) 2-methylheptane
B) 1-heptanol
C) heptanamine
D) heptanal
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following electromagnetic radiation has the longest wavelength?
A) UV
B) X-ray
C) IR
D) microwave
E) visible
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Compounds containing chlorine or bromine usually show a strong _____ peak.
A) molecular ion
B) base
C) M+1
D) M+2
E) all of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds will have odd m/z value for the molecular ion? 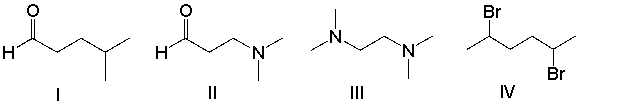
A) I
B) II
C) III
D) IV
E) all of them
Correct Answer

verified
Correct Answer
verified
Essay
How will you distinguish between the following compounds using high-resolution mass spectrometry? 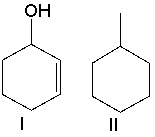
Correct Answer

verified
Compound I: m/z at 9...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following compounds is consistent with the following IR spectrum? 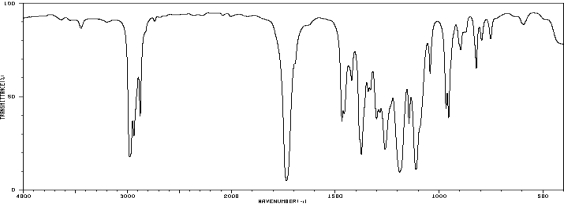 SDBS: National Institute of Advanced Industrial Science and Technology
SDBS: National Institute of Advanced Industrial Science and Technology 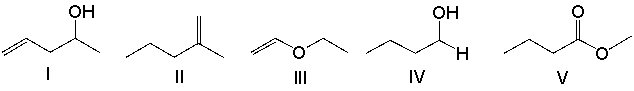
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds have the same degree of unsaturation? 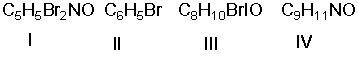
A) I and II
B) I and III
C) III and IV
D) II and III
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is expected to be the base peak in the mass spectrum of pentanal? 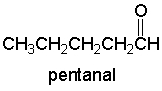
A) 29
B) 41
C) 44
D) 58
E) 86
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following compounds is consistent with the mass spectrum below? 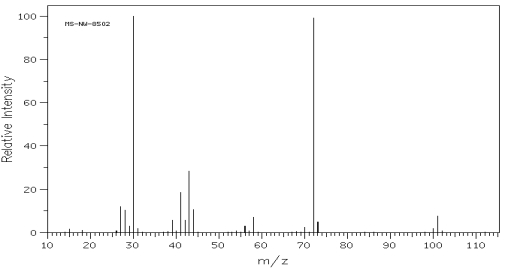 SDBS:
SDBS:
A) (CH3CH2CH2) 2CH
B) CH3CH2CHOHCH2CH2CH3
C) (CH3CH2CH2) 2O
D) (CH3CH2CH2) 2NH
E) CH3CH2CH2CH3
Correct Answer

verified
Correct Answer
verified
Essay
The C-O absorption in carboxylic acids appears around 1250 cm-1. The C-O absorption in an alcohol appears around 1050 cm-1. Explain why. 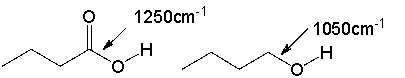
Correct Answer

verified
The C-O bond in a carboxylic a...View Answer
Show Answer
Correct Answer
verified
View Answer
Showing 41 - 60 of 135
Related Exams