Correct Answer

verified
Correct Answer
verified
Essay
Predict the product for the following Cope rearrangement and provide the curved arrow mechanism for the formation of the product. 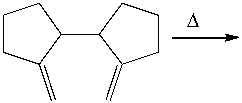
Correct Answer

verified
 _TB4454_00...
_TB4454_00...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which one of the following dienophiles is least reactive in the Diels-Alder reaction? 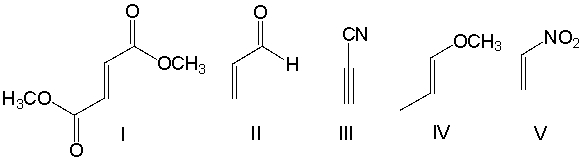
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Vitamin D3 has the following structure. Classify the bonds in vitamin D3 as conjugated, cumulated or isolated. 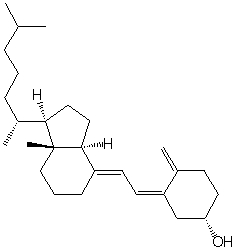
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following indicated C-C bonds is(are) the shortest? 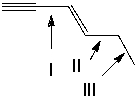
A) I
B) II
C) III
D) both I and II
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use Woodward-Fieser rules to estimate the max for the following compound. 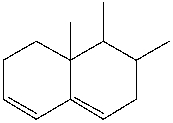
A) 238 nm
B) 233 nm
C) 222 nm
D) 229 nm
E) none of these
Correct Answer

verified
Correct Answer
verified
Essay
Provide the structure for 1,2 addition product for the following reaction and explain why it is a major product rather than 1,4 addition product. 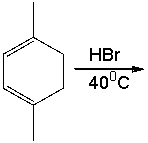
Correct Answer

verified
 _TB4454_00 This reaction is under thermo...
_TB4454_00 This reaction is under thermo...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Identify the pericyclic reaction in which two sigma bonds are formed and two pi bonds are broken.
A) sigmatropic rearrangement
B) cycloaddition reaction
C) electrolytic reaction
D) this is not a pericyclic reaction
Correct Answer

verified
Correct Answer
verified
Essay
Predict the major product for the following Diels-Alder reaction. 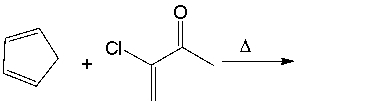
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds have conjugated double bonds? 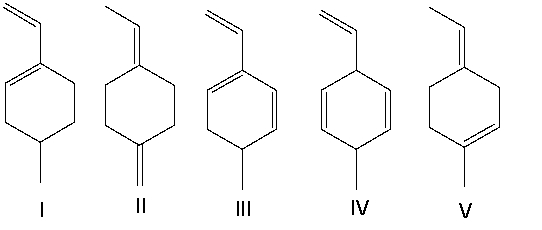
A) II and V
B) II, IV, and V
C) I and III
D) I, III, and IV
E) all of them
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following dienes is most stable?
A) CH3CH=CHCH=CHCH3
B) CH3CH=CHCH2CH=CH2
C) CH2=CHCH2CH2CH=CH2
D) CH2=CHCH(CH3) CH=CH2
E) CH3CH=C=CHCH2CH3
Correct Answer

verified
Correct Answer
verified
Essay
Predict the product for the following electrocyclic reaction. 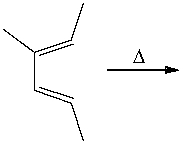
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 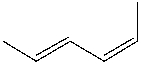
A) (2E, 4Z) -2,4-hexadiene
B) (2E, 4Z) -1,4-dimethyl-1,3-butadiene
C) (2Z, 4Z) -1,4-dimethyl-1,3-butadiene
D) (2Z, 4Z) -2,4-hexadiene
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds have isolated double bonds? 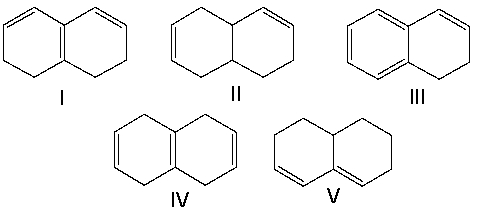
A) II and IV
B) III and V
C) I, III, and V
D) I and V
E) I and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes the stereochemistry of ring closure and the product for the following reaction? 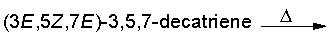
A) disrotatory, cis-5,6-diethyl-1,3-cyclohexadiene
B) conrotatory, cis-5,6-diethyl-1,3-cyclohexadiene
C) disrotatory, trans-5,6-diethyl-1,3-cyclohexadiene
D) conrotatory, trans -5,6-diethyl-1,3-cyclohexadiene
Correct Answer

verified
Correct Answer
verified
Essay
Predict the major product for the following Diels-Alder reaction. 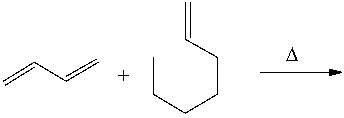
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following diene does not undergo Diels Alder reaction because _____. 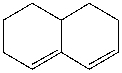
A) it does not have an electron donating group
B) it does not have an electron withdrawing group
C) the bicyclic ring does not function as a diene
D) it cannot adopt the s-cis conformation
E) the double bonds are not in the same cyclic ring
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the IUPAC name for the following compound? 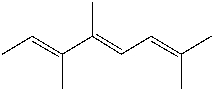
A) (2E,4Z,6E) -3,4,7,8-tetramethyl-2,4,6-heptatriene
B) (2Z,4E) -3,4,7-trimethyl-2,4,6-octatriene
C) (2E,4Z,6E) -2,5,6,7-tetramethyl-3,5,7-heptatriene
D) (2E,4Z) - 2,5,6-trimethyl-3,5,7-octatriene
E) (4E,6E) -2,5,6-trimethyl-2,4,6-octatriene
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which diene and dienophile would react to give the following Diels-Alder product? 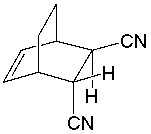
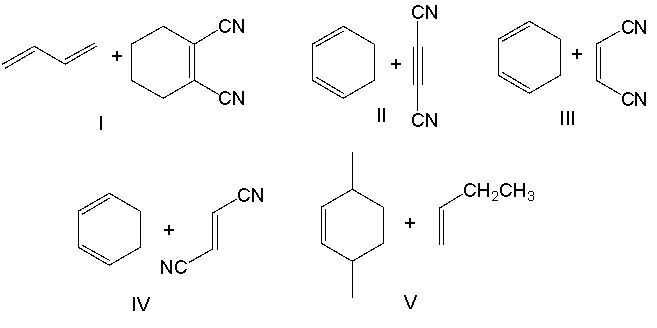
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes the stereochemistry of ring closure and the product for the following reaction? 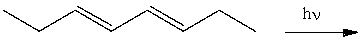
A) disrotatory, cis-3,4-diethylcyclobutene
B) conrotatory, cis-3,4-diethylcyclobutene
C) disrotatory, trans-3,4-diethylcyclobutene
D) conrotatory, trans -3,4-diethylcyclobutene
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 140
Related Exams