A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for TeBr2. 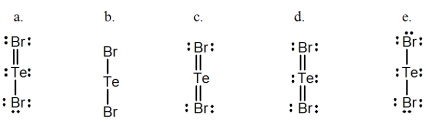
A) a
B) b
C) c
D) d
E) e
Correct Answer

verified
Correct Answer
verified
Essay
The Lewis structure of formaldehyde,CH2O,is shown.Use VSEPR theory to predict the molecular geometry and the H-C-H bond angle.Outline your reasoning. 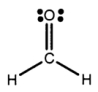
Correct Answer

verified
There are three electron groups around t...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
What is the shape of the PF3 molecule? Explain your answer,using VSEPR theory.
Correct Answer

verified
The Lewis structure has a lone pair on t...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the molecular shape of BCl3 as predicted by the VSEPR theory?
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
True/False
According to VSEPR theory,a molecule with the general formula AX3E2 (where E represents a lone pair on A)will be trigonal planar.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory,a molecule with the general formula AX2E2 will have a _____ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct Lewis structure for NOCl,a reactive material used as an ionizing solvent. 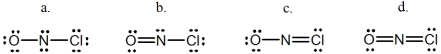
A) a
B) b
C) c
D) d
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a net dipole moment?
A) BeCl2
B) SF2
C) KrF2
D) CO2
E) CCl4
Correct Answer

verified
Correct Answer
verified
True/False
When resonance occurs,the bond lengths in a molecule fluctuate rapidly.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of ClCN as predicted by the VSEPR theory? (Carbon is the central atom. )
A) linear
B) bent
C) angular
D) trigonal
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formal charge on Cl in the structure shown for the perchlorate ion is 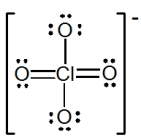
A) -2
B) -1
C) 0
D) +1
E) +2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of SCl3F as predicted by the VSEPR theory?
A) linear
B) bent
C) see-saw
D) T-shaped
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Hydrazine,N2H4,is a good reducing agent that has been used as a component in rocket fuels.Select its Lewis structure. 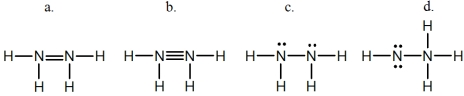
A) a
B) b
C) c
D) d
E) None of these choices is correct.
Correct Answer

verified
Correct Answer
verified
Showing 81 - 94 of 94
Related Exams