A) Ozone normally forms in the upper atmosphere.
B) Chlorofluorocarbons deplete the ozone layer.
C) Ozone protects life on Earth from harmful ultraviolet light.
D) None of the above statements are true.
E) All the of the first three statements are true.
Correct Answer

verified
Correct Answer
verified
True/False
A precipitation reaction occurs when water is formed as a product.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT an acid?
A) HClO4
B) HNO3
C) HC2H3O2
D) H2SO4
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is NOT evidence that a chemical reaction has occurred?
A) color change when chemicals are contacted with each other
B) solid formation when chemicals are contacted with each other
C) gas formation when chemicals are contacted with each other
D) emission of light when chemicals are contact with each other
E) All of the above are evidence of a chemical reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Considering the following precipitation reaction: Pb(NO3) 2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq) Which ion(s) would NOT be present in the net ionic equation?
A) Pb2+,NO3-
B) K+,NO3-
C) K+, Pb2+
D) K+, I-
E) All the above ions are in the net ionic equation.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the net ionic equation for the reaction of hydrochloric acid with potassium hydroxide?
A) H+ + Cl- + K+ + OH- → H2O + K+ + Cl-
B) HCl + KOH → H2O + KCl
C) H+ + OH- → H2O
D) 2H+ +2Cl- + K2+ + 2OH- → H2O + K2+ +2 Cl-
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What are the coefficients for the following reaction when it is properly balanced? ___potassium iodide + ___lead (II) acetate → ___lead (II) iodide +___potassium acetate
A) 2,1,1,1
B) 2,1,1,2
C) 3,2,2,1
D) 1,1,2,2
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the equation __Ca3N2 + __H2O → __Ca(OH) 2 + __NH3 is balanced,the coefficient of H2O is:
A) 2
B) 3
C) 6
D) 12
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
A precipitate will form when you mix solutions of potassium chloride and lead nitrate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A redox reaction has occurred ________.
A) when a substance reacts with elemental oxygen
B) when an alkali metal reacts with an alkaline earth metal
C) when one substance transfers protons to another
D) when one substance forms a solid
E) when all of the above occur
Correct Answer

verified
Correct Answer
verified
True/False
A strong electrolyte solution contains ionic compounds that completely dissociate in water.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Considering the following precipitation reaction: Pb(NO3) 2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq) What is the correct complete ionic equation?
A) Pb2+ + (NO3) 2- + 2K+ + 2I- → PbI2(s) + 2K+ + 2NO3-
B) Pb2+ + 2NO3- +2K+ + I- → PbI2(s) + 2K+ + NO3-
C) Pb2+ + 2NO3- + 2K+ + 2I- → PbI2(s) + 2K+ + 2NO3-
D) Pb2+ + 2NO3- + 2K+ + 2I- → Pb2+ + 2I- + 2K+ + 2NO3-
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of a reaction occurs when a sodium hydroxide solution is mixed with an acetic acid solution?
A) precipitation
B) acid-base neutralization
C) oxidation-reduction
D) gas evolution
E) no reaction
Correct Answer

verified
Correct Answer
verified
True/False
The net ionic equation for the reaction of sodium hydroxide plus hydrochloric acid is 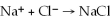 .
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The mixing of sodium metal and chlorine gas would be the type of reaction known as:
A) oxidation-reduction
B) gas evolution
C) neutralization
D) precipitation
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of reaction is the generic equation  ?
?
A) synthesis/combination
B) decomposition
C) single displacement
D) double-displacement
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Cl-,Br-,and I- are mostly insoluble.
Correct Answer

verified
Correct Answer
verified
True/False
A precipitation reaction will occur when sodium chloride is mixed with potassium nitrate.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the gas produced when hydrochloric acid is reacted with ammonium carbonate?
A) ammonia
B) chlorine
C) hydrogen
D) carbon dioxide
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
When balancing a chemical equation you may alter the coefficients but not the subscripts in the equation.
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 113
Related Exams