Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pressure of 760 mm Hg when expressed in units of in Hg?
A) 29.92 in Hg
B) 101,325 in Hg
C) 760 in Hg
D) 1 in Hg
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the pressure of a 3.00 L gas vessel that has 18.0 grams of helium at 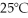 ?
(R= 0.0821 L atm/ mol K)
?
(R= 0.0821 L atm/ mol K)
A) 147 atm
B) 36.7 atm
C) 32.6 atm
D) 1.81 atm
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A sample of helium gas initially at 37.0°C,785 torr and 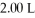 Was heated to 58.0°C while the volume expanded to
Was heated to 58.0°C while the volume expanded to 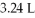 .What is the final pressure in atm?
.What is the final pressure in atm?
A) 517
B) 0.681
C) 1.79
D) 3.21
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the final pressure (expressed in atm) of a 3.05 L system initially at 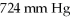 And
And 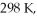 That is compressed to a final volume of
That is compressed to a final volume of 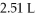 At
At 
A) 1.06
B) 1.60
C) 806
D) 860
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a mixture of gases contained 78% nitrogen at a pressure of 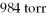 And 22% carbon dioxide at
And 22% carbon dioxide at 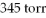 ,what is the total pressure of the system?
,what is the total pressure of the system?
A) 1,329 atm
B) 17.5 cm Hg
C) 639 torr
D) 1.75 atm
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is TRUE for gases? 1.The temperature of a gas is inversely proportional to its pressure. 2.The volume of a gas is directly proportional to the pressure in torr. 3.The pressure of a gas is due to collisions of the gas molecules.
A) 1 only
B) 2 only
C) 3 only
D) 1 and 2 only
E) 1 and 3 only
Correct Answer

verified
Correct Answer
verified
Multiple Choice
1 torr is equal to:
A) 760 mm Hg.
B) 1 mm Hg.
C) 1 Pa.
D) 14.7 psi.
E) all of the above
Correct Answer

verified
Correct Answer
verified
True/False
If the number of gas particles is halved,the volume of the gas will be halved given that the temperature and pressure do not change.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the major component of the air we breathe?
A) nitrogen
B) oxygen
C) argon
D) carbon dioxide
E) smog
Correct Answer

verified
Correct Answer
verified
True/False
If the column of mercury in a barometer drops to a lower reading,this means the measured pressure has decreased.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 5.00 liter balloon of gas at 25°C is cooled to 0°C.What is the new volume (liters) of the balloon?
A) 0 liters
B) 22.4 liters
C) 5.46 liters
D) 4.58 liters
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
Gas particles act independently of each other.
Correct Answer

verified
Correct Answer
verified
True/False
Gases are a collection of particles in constant,unpredictable motion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the temperature (°C) of 2.48 moles of gas stored in a 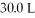 Container at
Container at 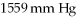 ?
(R= 0.0821 L atm/ mol K)
?
(R= 0.0821 L atm/ mol K)
A) 302
B) 189
C) 29
D) -84
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the proper form of the combined gas law?
A) ![]()
=
![]()
B) ![]()
=
![]()
C) ![]()
=
![]()
D) ![]()
![]()
![]()
=
![]()
![]()
![]()
E) none of the above
Correct Answer

verified
Correct Answer
verified
True/False
The volume of a gas is independent of the temperature.
Correct Answer

verified
Correct Answer
verified
True/False
For all gas law calculations,the temperature must be in kelvins.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the initial temperature (°C) of a system that has the pressure decreased by 10 times while the volume increased by 5 times with a final temperature of 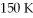 ?
?
A) 27
B) 75
C) -198
D) 300
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Boyle's Law is expressed as:
A) V is proportional to ![]()
B) P is proportional to V
C) V is proportional to ![]()
D) V is proportional to T
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 81 - 100 of 122
Related Exams