A) A
B) B
C) C
D) D
E) E
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the best Lewis structure for ClCN.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
True/False
In formaldehyde, CH2O, both the formal charge and the oxidation number of carbon are zero.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following structures does the central atom have a formal charge of +2?
A) SF6 ![]()
B) SO42- ![]()
C) O3 ![]()
D) BeCl2 ![]()
E) AlCl4- ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of BeH2 as predicted by the VSEPR theory?
A) linear
B) bent
C) angular
D) trigonal
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the molecular shape of NOCl as predicted by the VSEPR theory? 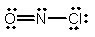
A) linear
B) trigonal planar
C) bent
D) tetrahedral
E) trigonal pyramidal
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use VSEPR theory to decide which one of the following molecules and ions will definitely have at least one 90° bond angle in it. (In each case except water, the central atom is the first one in the formula.)
A) AlCl4-
B) NH3
C) PCl5
D) CO2
E) H2O
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following is the best Lewis structure a resonance structure? (central atoms are bold)
A) CO2
B) ClO3-
C) COCl2
D) NO2+
E) HCN
Correct Answer

verified
Correct Answer
verified
True/False
In order for a non-cyclic triatomic molecule to be bent, VSEPR theory requires that there must be two lone pairs on the central atom.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The formal charges on Cl and O in the structure shown for the ClO- ion are, respectively 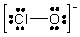
A) 0 and -1
B) -1 and 0
C) 1 and -2
D) -2 and 1
E) none of the above
Correct Answer

verified
Correct Answer
verified
Essay
Draw Lewis structures, showing all valence electrons, for: a. N b. Br- c. O2 d. SO42-
Correct Answer

verified
A)11ec6d40_88db_23e6_9cd1_6949936dcf2d_T...View Answer
Show Answer
Correct Answer
verified
View Answer
True/False
Bond angles of 180° only occur around atoms which display linear molecular geometry.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which one of the following species is the central atom (the first atom in the formula) an exception to the octet rule?
A) NH3
B) NH4+
C) I2
D) BH4-
E) SF6
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the ideal bond angles around carbon in C2I2 using the molecular shape given by the VSEPR theory.
A) 90°
B) 109°
C) 120°
D) 180°
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX5 will have a ______ molecular shape.
A) tetrahedral
B) trigonal planar
C) trigonal pyramidal
D) trigonal bipyramidal
E) see-saw
Correct Answer

verified
Correct Answer
verified
Essay
List the three important ways in which molecules can violate the octet rule, and in each case draw one Lewis structure of your choice as an example.
Correct Answer

verified
Electron-deficient molecules have fewer ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
What is the molecular shape of the thiocyanate anion, SCN-, as predicted by the VSEPR theory? (Carbon is the central atom.)
A) linear
B) bent
C) angular
D) trigonal
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Thionyl chloride is used as an oxidizing and chlorinating agent in organic chemistry. Select the best Lewis structure for SOCl2.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) None of the above structures is suitable for SOCl2.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX2E2 will have a _____ molecular shape.
A) linear
B) bent
C) trigonal planar
D) tetrahedral
E) see-saw
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to VSEPR theory, a molecule with the general formula AX3E2 will have a _____ molecular shape.
A) trigonal pyramidal
B) trigonal bipyramidal
C) trigonal planar
D) T-shaped
E) see-saw
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 109
Related Exams