A) positron: ![]()
B) neutron: ![]()
C) helium-3: ![]()
D) alpha particle: ![]()
E) proton: ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Fill in missing sub- and superscripts for all particles to complete the following equation for positron decay. 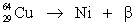
Correct Answer

verified
Correct Answer
verified
True/False
The (negative) binding energy per nucleon reaches a maximum for the isotope 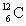 .
.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The radioisotope 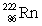 will decay through
will decay through
A) . decay.
B) . decay.
C) . decay.
D) positron decay.
E) electron capture.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope with a low value of N/Z will generally decay through
A) . decay.
B) . decay.
C) . decay.
D) electron capture.
E) spontaneous fission.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 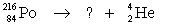
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 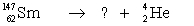
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 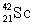 is unstable. This is predictable because
is unstable. This is predictable because
A) the number of neutrons is too large in relation to the number of protons.
B) the number of neutrons is too small in relation to the number of protons.
C) the atomic number is too large.
D) the mass number is too large.
E) Sc isotopes are all unstable.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 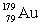 has a half-life of 7.5 seconds. If a sample contains 144 atoms of
has a half-life of 7.5 seconds. If a sample contains 144 atoms of 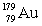 , approximately how many such atoms were there present 30 seconds earlier?
, approximately how many such atoms were there present 30 seconds earlier?
A) 576
B) 1152
C) 2304
D) 4320
E) 4.30 × 108
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 7.85 × 10-5 mol sample of copper-61 emits 1.47 × 1019 positrons in 90.0 minutes. What is the decay constant for copper-61?
A) 0.00230 h-1
B) 0.00346 h-1
C) 0.207 h-1
D) 0.311 h-1
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
All the disintegrations of a sample of an unknown nuclide weighing 4.6 × 10-2 g were counted. In the first half-life of the sample, the total number of disintegrations counted was 4.3 × 1020. What is the atomic weight of the unknown element?
A) 32 amu
B) 16 amu
C) 8 amu
D) 4 amu
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An isotope with Z > 83, which lies close to the band of stability, will generally decay through
A) . decay.
B) . decay.
C) . decay.
D) positron decay.
E) electron capture.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 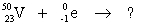
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Carbon-14 will emit a particle with an energy of 0.1565 MeV. What is this energy in joules?
A) 1.0 × 10-24 J
B) 2.5 × 10-20 J
C) 1.0 × 10-18 J
D) 2.5 × 10-14 J
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A N-14 nucleus is hit by a particle, forming a C-14 nucleus and a proton as the only products. Identify the type of particle which struck the N-14 nucleus.
A) alpha
B) proton
C) electron
D) neutron
E) deuterium
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A certain isotope has a specific activity of 7.29 × 10-4 Ci/g. How many particles will a 75.0 mg sample emit in one hour?
A) 9.99 × 104
B) 2.02 × 106
C) 7.28 × 109
D) 1.29 × 1012
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The isotope 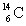 is unstable. This is predictable because
is unstable. This is predictable because
A) N/Z 1.
B) N/Z is relatively low and Z < 20.
C) N/Z is relatively large and Z < 20.
D) Z is small.
E) N is large.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the nuclide that completes the following nuclear reaction. 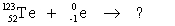
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) none of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following types of radioactive decay does not produce new element?
A) gamma emission
B) electron capture
C) beta emission
D) alpha emission
E) double beta emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Iodine-131, t1/2 = 8.0 days, is used in diagnosis and treatment of thyroid gland diseases. If a laboratory sample of iodine-131 initially emits 9.95 × 1018 particles per day, how long will it take for the activity to drop to 6.22 × 1017 particles per day?
A) 2.0 days
B) 16 days
C) 32 days
D) 128 days
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 94
Related Exams