A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What would be the major product,B,of the following reaction sequence? 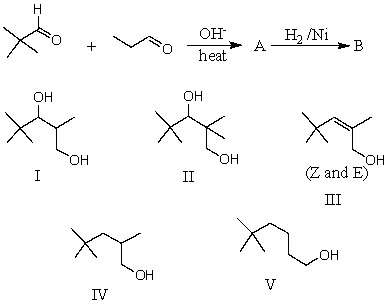
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What starting compound(s) would you use in an aldol reaction to prepare as the major product: 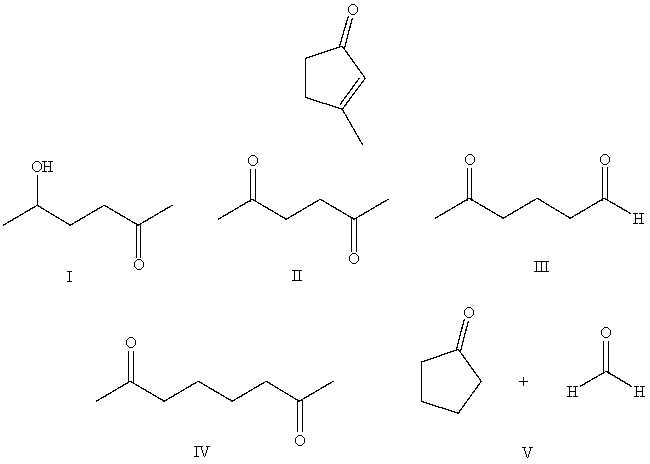
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which compound may be prepared using a Mannich reaction? 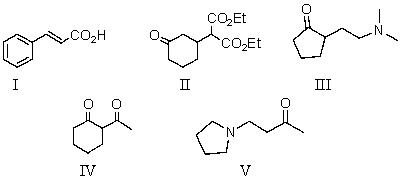
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Essay
Suggest a reasonable synthetic strategy to carry out the following transformation. 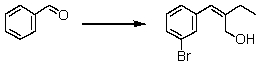
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the missing reagent? 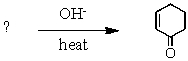
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
What is the final product of the following reaction sequence? Give structural details of all significant intermediates. 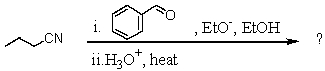
Correct Answer

verified
Correct Answer
verified
Essay
What is the product of the following reaction? 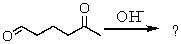
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What starting compound(s) would you use in an aldol reaction to prepare as the major product: 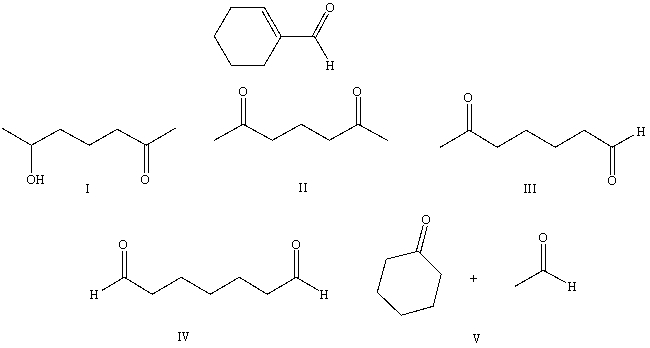
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of these compounds can react with 4-methylhexanal to afford good yields of the crossed aldol product?
A) 3-(4-Nitrophenyl) propanal
B) 2-Methyl-3-pentanone
C) 2-(4-Nitrophenyl) propanal
D) 3-(4-Nitrophenyl) -2-butanone
E) None of the above compounds will give good yields of the crossed aldol product with 4-methylhexanal.
Correct Answer

verified
Correct Answer
verified
Essay
Explain why diethyl pentanedioate does not undergo a Dieckmann condensation.
Correct Answer

verified
Dieckmann condensation of diethyl pentan...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
What is the final product of the following reaction sequence? Give structural details of all significant intermediates. 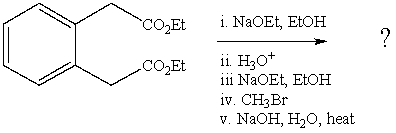
Correct Answer

verified
Correct Answer
verified
Essay
An aldol reaction that starts with two different carbonyl compounds is called a _______________.
Correct Answer

verified
crossed al...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Explain why ethyl 2-methylpropanoate does not undergo the usual Claisen condensation.
Correct Answer

verified
In order for Claisen condensation to occ...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
The product,C,of the following sequence of reactions, 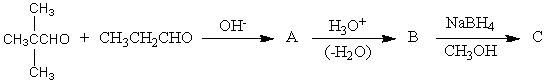 would be:
would be:
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the product of the Dieckmann-like condensation of this ketoester, 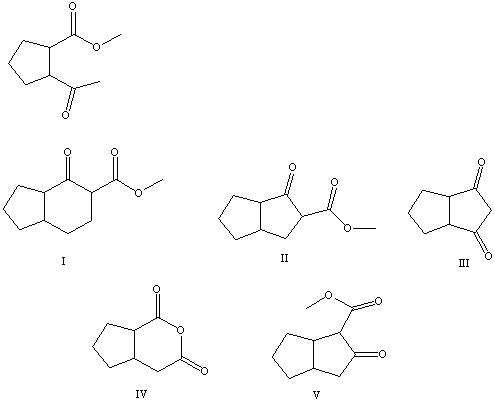
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the product from the following sequence: 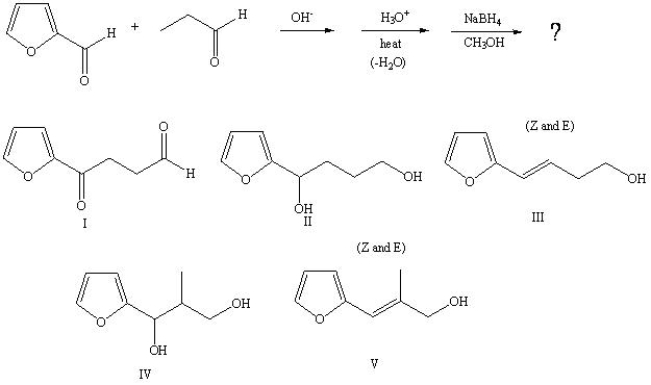
A) I
B) II
C) III
D) IV
E) V
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Why is CH3ONa not used in the Claisen condensation of ethyl acetate?
A) CH3O- is a weaker base than the CH3CH2O- which is used.
B) CH3O-Na+ is more difficult to prepare than CH3CH2O-Na+.
C) CH3O- would abstract a proton from the ethyl group of the ester.
D) Use of CH3O-Na+ would result in transesterification.
E) CH3O-Na+ can be used as well as CH3CH2O-Na+.
Correct Answer

verified
Correct Answer
verified
Essay
There are several possible cyclization products that might be formed when 5-methyl-6-oxoheptanal undergoes intramolecular aldol condensation in presence of ethoxide.Draw the structures of all these possible products.Comment on which of these is likely to be the major product,clearly explaining your rationale.
Correct Answer

verified
There are 3 possible products (I-III)tha...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
What is the final product of the following reaction sequence? Give structural details of all significant intermediates. 
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 131
Related Exams