Correct Answer

verified
Correct Answer
verified
Multiple Choice
If lung problems like emphysema occur,the blood pH of the patient is expected to ________.
A) saturate
B) increase
C) decrease
D) stay the same
E) concentrate
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A chemical reaction has reached equilibrium when ________.
A) the concentrations of reactants and products are equal
B) all reactants have been converted to products
C) all products have been removed from the reaction mixture
D) the catalyst has been used up
E) the rate of the forward reaction equals the rate of the reverse reaction
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The neutralization reaction between 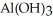 and
and 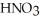 produces the salt with the formula ________.
produces the salt with the formula ________.
A) ![]() O
O
B) ![]()
C) ![]()
D) ![]() ) 3
) 3
E) ![]() OH
OH
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When hyperventilation (rapid breathing) causes a patient to exhale large amounts of 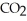 ,the blood pH rises in a condition called ________.
,the blood pH rises in a condition called ________.
A) metabolic acidosis
B) metabolic alkalosis
C) respiratory acidosis
D) respiratory alkalosis
E) pulmonary distress
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements correctly describes the hydronium-hydroxide balance in the given solution?
A) In acids,[ ![]() ] is greater than [
] is greater than [ ![]()
![]() ].
].
B) In bases,[ ![]() ] = [
] = [ ![]()
![]() ].
].
C) In neutral solutions,[ ![]()
![]() ] = [
] = [ ![]() O].
O].
D) In bases,[ ![]() ] is greater than [
] is greater than [ ![]()
![]() ].
].
E) In bases,[ ![]() ] is less than [
] is less than [ ![]()
![]() ].
].
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Ammonium hydroxide is a weak base because ________.
A) it forms a dilute solution
B) it is only slightly soluble in water
C) is a poor acceptor of protons
D) it dissociates only slightly in water
E) it is completely ionized in aqueous solution
Correct Answer

verified
Correct Answer
verified
Showing 61 - 67 of 67
Related Exams