A) -2
B) -1
C) +1
D) two of these
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following: F2,B2,O2,N2 ,are paramagnetic?
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tetracyanoethylene has the skeleton shown below: 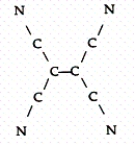 From its Lewis structure determine the following:
-How many of the atoms are sp2 hybridized?
From its Lewis structure determine the following:
-How many of the atoms are sp2 hybridized?
A) 2
B) 4
C) 6
D) 8
E) 10
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the carbon atom in the cation CH3+ is:
A) sp2
B) sp3
C) dsp
D) sp
E) none of these
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Which of these statements about benzene is true?
A) All carbon atoms in benzene are sp3 hybridized.
B) Benzene contains only bonds between C atoms.
C) The bond order of each C-C bond in benzene is 1.5.
D) Benzene is an example of a molecule that displays ionic bonding.
E) All of these statements are false.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many unpaired electrons in the F22+ ion are based on molecular orbital theory? The order of the molecular orbitals are ( 2s) ( *2s) ( 2p) ( 2p) ( *2p) ( *2p) .
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Short Answer
__________ causes a substance to be attracted into the inducing magnetic field.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about the molecule BN is false?
A) It is paramagnetic.
B) Its bond order is 2.
C) The total number of electrons is 12.
D) It has two pi bonds.
E) All of these are true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the central atom in XeF5+ is:
A) sp
B) sp2
C) sp3
D) dsp3
E) d2sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For how many of the following does bond order decrease if you take away one electron from the neutral molecule? B2,C2,P2,F2
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the shortest bond length?
A) O22-
B) O2
C) O2-
D) O2+
E) Two of these have the shortest bond length.
Correct Answer

verified
D
Correct Answer
verified
Short Answer
The number of molecular orbitals formed is always __________ the number of atomic orbitals combined.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the central atom in ClF2+ is:
A) sp
B) sp2
C) sp3
D) dsp3
E) d2sp3
Correct Answer

verified
C
Correct Answer
verified
Essay
Consider three molecules - A,B,C.Molecule A has a hybridization of sp3.Molecule B has two more effective pairs (electron pairs around the central atom)than molecule A.Molecule C consists of one bond and two bonds.Give the molecular structure,hybridization,bond angles,and an example for each molecule.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Sulfur trioxide is known to be planar with all the oxygen atoms equidistant from the central sulfur atom.On the basis of these facts,which of the following conclusions may be drawn concerning this molecule? I.It can be represented by three equivalent resonance structures. II.The dipoles associated with each S-O bond are equal in magnitude. III.The sulfur atom is sp2 hybridized.
A) I only
B) II only
C) III only
D) I and II only
E) I,II,and III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The hybridization of the central atom,Al,in AlBr3 is
A) sp
B) sp2
C) sp3
D) dsp3
E) d2sp3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tetracyanoethylene has the skeleton shown below:  From its Lewis structure determine the following:
-How many sigma and pi bonds are in the molecule?
From its Lewis structure determine the following:
-How many sigma and pi bonds are in the molecule?
A) 4 sigma and 5 pi
B) 6 sigma and 8 pi
C) 9 sigma and 8 pi
D) 9 sigma and 9 pi
E) 5 sigma and 8 pi
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is false?
A) C2 is paramagnetic.
B) C2 is diamagnetic.
C) The carbon-carbon bond in C22- is stronger than the one in CH3CH3.
D) The carbon-carbon bond in C22- is shorter than the one in CH3CH3.
E) Two of the above.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many electrons are involved in pi bonding in benzene,C6H6?
A) 12
B) 30
C) 3
D) 6
E) 18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the molecules below to answer the next three questions. 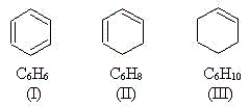 -Which molecule(s) have equivalent C-C bonds throughout the molecule?
-Which molecule(s) have equivalent C-C bonds throughout the molecule?
A) I
B) II
C) III
D) all of the above
E) none of the above
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 101
Related Exams