A) 5.26 103 mL
B) 10.7 mL
C) 10.4 mL
D) 18.5 mL
E) 6.02 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A mixture of BaCl2 and NaCl is analyzed by precipitating all the barium as BaSO4.After addition of an excess of Na2SO4 to a 3.725-g sample of the mixture,the mass of precipitate collected is 2.734 g.What is the mass percentage of barium chloride in the mixture?
A) 82.28%
B) 73.40%
C) 43.18%
D) 65.47%
E) 19.60%
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You have exposed electrodes of a light bulb in a solution of H2SO4 such that the light bulb is on.You add a dilute solution and the bulb grows dim.Which of the following could be in the solution?
A) Ba(OH) 2
B) NaNO3
C) K2SO4
D) Cu(NO3) 2
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When solutions of carbonic acid and potassium hydroxide react,which of the following are NOT present in the complete ionic equation?
A) hydrogen ion
B) carbonate ion
C) potassium ion
D) hydroxide ion
E) water
Correct Answer

verified
Correct Answer
verified
Essay
Write balanced equations for each of the processes,choosing from the following substances as reactants:
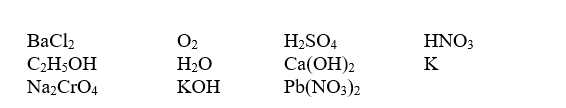 -Precipitation of BaSO4 from solution
-Precipitation of BaSO4 from solution
Correct Answer

verified
H2SO...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Which of the following ions is most likely to form an insoluble sulfate?
A) K+
B) Li+
C) Ca2+
D) S2-
E) Cl-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A 74.28-g sample of Ba(OH) 2 is dissolved in enough water to make 2.450 liters of solution.How many mL of this solution must be diluted with water in order to make 1.000 L of 0.100 M Ba(OH) 2?
A) 565 mL
B) 177 mL
C) 17.7 mL
D) 4.34 mL
E) 231 mL
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many of the following are oxidation-reduction reactions? NaOH + HCl NaCl + H2O Cu + 2AgNO3 2Ag + Cu(NO3) 2 Mg(OH) 2 MgO + H2O N2 + 3H2 2NH3
A) 0
B) 1
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The net ionic equation contains which of the following terms?
A) Ag+(aq)
B) Ba2+(aq)
C) NO3- (aq)
D) H+ (aq)
E) AgCl(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following reactions does not involve oxidation-reduction?
A) CH4 + 3O2 2H2O + CO2
B) Zn + 2HCl ZnCl2 + H2
C) 2Na + 2H2O 2NaOH + H2
D) MnO2 + 4HCl Cl2 + 2H2O + MnCl2
E) All are oxidation-reduction reactions.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Aqueous solutions of barium chloride and silver nitrate are mixed to form solid silver chloride and aqueous barium nitrate. -The balanced molecular equation contains which one of the following terms?
A) AgCl (s)
B) 2AgCl (s)
C) 2Ba(NO3) 2 (aq)
D) BaNO3 (aq)
E) 3AgCl (aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An unknown diprotic acid requires 44.39 mL of 0.111 M NaOH to completely neutralize a 0.580-g sample.Calculate the approximate molar mass of the acid.
A) 406 g/mol
B) 235 g/mol
C) 118 g/mol
D) 59 g/mol
E) 203 g/mol
Correct Answer

verified
Correct Answer
verified
Essay
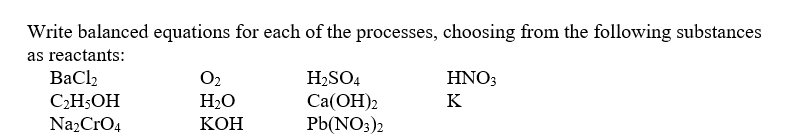 -Formation of hydrogen gas
-Formation of hydrogen gas
Correct Answer

verified
2K + HView Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
In the balanced molecular equation for the neutralization of sodium hydroxide with sulfuric acid,the products are:
A) NaSO4 + H2O
B) NaSO3 + 2H2O
C) 2NaSO4 + H2O
D) Na2S + 2H2O
E) Na2SO4 + 2H2O
Correct Answer

verified
Correct Answer
verified
Short Answer
A molecule with an unequal charge distribution is said to be a __________ molecule.
Correct Answer

verified
Correct Answer
verified
Essay
Selecting from the following reagents,indicate which reagents would be mixed to give the compounds described. CuSO4(aq) Fe2(CO3)3(s) NH3(aq) CuCO3(s) FeCl3(aq) Na2SO4(aq) Cr(OH)3(s) H2SO4(aq) -FeCl3(aq)+ Na2SO4(aq)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You mix 55 mL of 1.00 M silver nitrate with 25 mL of 0.84 M sodium chloride.What mass of silver chloride should you form?
A) 3.0 g
B) 6.0 g
C) 3.3 g
D) 6.6
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following unbalanced oxidation-reduction reaction: Fe2+ + Br2 Fe3+ + Br- In the balanced equation,the number of electrons transferred is
A) 1
B) 3
C) 2
D) 4
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reactions: Pb2+ + 2I- PbI2 2Ce4+ + 2I- I2 + 2Ce3+ HOAc + NH3 NH4+ + OAc- Are examples of
A) acid-base reactions
B) unbalanced reactions
C) precipitation,acid-base,and redox reactions,respectively
D) redox,acid-base,and precipitation reactions,respectively
E) precipitation,redox,and acid-base reactions,respectively
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The oxidation state of iodine in IO3- is:
A) 0
B) +3
C) -3
D) +5
E) -5
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 117
Related Exams