A) 0
B) 1
C) 2
D) 3
E) 4
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
A general reaction written as A + 2B C + 2D is studied and yields the following data:
 -What is the overall order of the reaction?
-What is the overall order of the reaction?
A) 0
B) 1
C) 2
D) 3
E) 4
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about enzymes is incorrect?
A) They are proteins that catalyze specific biologic reactions.
B) Several hundred are now known.
C) The molecules they react with are called substrates.
D) They are equal to inorganic catalysts in efficiency.
E) All of these are correct.
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
At a given temperature,a first-order reaction has a rate constant of 3.4 10-3 s-1.The time required for the reaction to be 44% completed is
A) 4.0 min
B) 1.2 min
C) 20 min
D) 2.8 min
E) 19 min
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which step is rate determining?
A) both steps
B) step 1
C) step 2
D) a step that is intermediate to step 1 and step 2
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction aA Products,use the following choices -The half-life is constant.
A) zero order in A
B) first order in A
C) second order in A
D) a,b,c
E) none of the above
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of this reaction is approximately
A) 15 minutes
B) 18 minutes
C) 23 minutes
D) 36 minutes
E) 45 minutes
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the temperature changed to 310 K,the rate constant k would change.The ratio of k at 310 K to k at 300.0 K is closest to what whole number?
A) 1
B) 2
C) 3
D) 4
E) 5
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction A B + C is known to be zero order in A with a rate constant of 5.0 10-2 mol/L·s at 25°C.An experiment was run at 25°C where [A]0 = 1.0 10-3 M. -The integrated rate law is
A) [A] = kt
B) [A] - [A]0 = kt
C) ![]()
D) ![]()
E) [A]0 - [A] = kt
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to the proposed mechanism,what should the overall rate law be?
A) rate = k[A2]2
B) rate = k[A2]
C) rate = k[A2][B2]
D) rate = k[A2][R]
E) rate = k[R]2
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
According to collision theory,the activated complex that forms in step 1 could have which of the following structures? (The dotted lines represent partial bonds. )
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is typically true for a catalyst? I.The concentration of the catalyst will go down as a reaction proceeds. II.The catalyst provides a new pathway in the reaction mechanism. III.The catalyst speeds up the reaction.
A) I only
B) II only
C) III only
D) I and III
E) II and III
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The kinetics of the reaction ![The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of n? A) 0 B) 0.5 C) 1 D) 1.5 E) 2](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66f8_f38e_93a6_31a6fa3982fe_TB6423_11_TB6423_11_TB6423_11.jpg) were studied and the following results obtained,where the rate law is:
were studied and the following results obtained,where the rate law is: ![The kinetics of the reaction were studied and the following results obtained,where the rate law is: For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. For a run where [A]<sub>0</sub> = 1.0 \times 10<sup>-</sup><sup>3</sup> M and [B]<sub>0</sub> = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 \times 10<sup>-</sup><sup>2</sup> s<sup>-</sup><sup>1</sup>. -What is the value of n? A) 0 B) 0.5 C) 1 D) 1.5 E) 2](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66f8_f38f_93a6_512646056c97_TB6423_11_TB6423_11_TB6423_11.jpg) For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-What is the value of n?
For a run where [A]0 = 1.0 10-3 M and [B]0 = 5.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -5.0 10-2 s-1.
For a run where [A]0 = 1.0 10-3 M and [B]0 = 10.0 M,a plot of ln [A] versus t was found to give a straight line with slope = -7.1 10-2 s-1.
-What is the value of n?
A) 0
B) 0.5
C) 1
D) 1.5
E) 2
G) C) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: 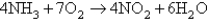 At a certain instant the initial rate of disappearance of the oxygen gas is X.What is the value of the appearance of water at the same instant?
At a certain instant the initial rate of disappearance of the oxygen gas is X.What is the value of the appearance of water at the same instant?
A) 1.2 X
B) 1.1 X
C) 0.86 X
D) 0.58 X
E) cannot be determined from the data
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following initial rate data were found for the reaction
2MnO4- + 5H2C2O4 + 6H+ 2Mn2+ + 10CO2 + 8H2O
 -What is the value of the rate constant?
-What is the value of the rate constant?
A) 2 105 M.s-1
B) 2 105 M-2.s-1
C) 200 M-1.s-1
D) 200 M-2.s-1
E) 2 10-4 M.s-1
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Of what use is it to find a rate law for a reaction?
A) We can use the rate law to directly determine coefficients in the balanced equation.
B) From the rate law we can evaluate potential reaction mechanisms.
C) The rate law gives us a good indication of the thermodynamic stability of the products.
D) The rate law can lead us to determine the equilibrium constant for the reaction.
E) None of these.
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction 2NO + O2 2NO2 obeys the rate law - ![The reaction 2NO + O<sub>2</sub> \to 2NO<sub>2</sub> obeys the rate law - = k<sub>obsd</sub>[NO]<sup>2</sup>[O<sub>2</sub>]. Which of the following mechanisms is consistent with the experimental rate law? A) NO + NO \to N<sub>2</sub>O<sub>2</sub> (slow) N<sub>2</sub>O<sub>2</sub> + O<sub>2</sub> \to 2NO<sub>2 </sub> (fast) B) NO + O<sub>2</sub> <sub> </sub> NO<sub>3</sub> (fast equilibrium) NO<sub>3</sub> + NO \to 2NO<sub>2 </sub> (slow) C) 2NO N<sub>2</sub>O<sub>2</sub> (fast equilibrium) N<sub>2</sub>O<sub>2</sub> \to NO<sub>2</sub> + O (slow) NO + O \to NO<sub>2</sub> (fast) D) O<sub>2</sub> + O<sub>2</sub> \to O<sub>2</sub> + O<sub>2 </sub> (slow) O<sub>2</sub> + NO \to NO<sub>2</sub> + O (fast) O + NO \to NO<sub>2 </sub> (fast) E) none of these](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66fc_9d4c_93a6_c912ba068099_TB6423_11.jpg) = kobsd[NO]2[O2]. Which of the following mechanisms is consistent with the experimental rate law?
= kobsd[NO]2[O2]. Which of the following mechanisms is consistent with the experimental rate law?
A) NO + NO N2O2 (slow) N2O2 + O2 2NO2 (fast)
B) NO + O2 ![]() NO3 (fast equilibrium) NO3 + NO 2NO2 (slow)
NO3 (fast equilibrium) NO3 + NO 2NO2 (slow)
C) 2NO ![]() N2O2 (fast equilibrium) N2O2 NO2 + O (slow) NO + O NO2 (fast)
N2O2 (fast equilibrium) N2O2 NO2 + O (slow) NO + O NO2 (fast)
D) O2 + O2 O2 + O2 (slow) O2 + NO NO2 + O (fast) O + NO NO2 (fast)
E) none of these
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The reaction: 2A + B C
Has the following proposed mechanism:
Step 1: A + B ![The reaction: 2A + B \to C Has the following proposed mechanism: Step 1: A + B D (fast equilibrium) Step 2: D + B \to E Step 3: E + A \to C + B If step 2 is the rate-determining step,then the rate of formation of C should equal: A) k[A] B) k[A]<sup>2</sup>[B] C) k[A]<sup>2</sup>[B]<sup>2</sup> D) k[A][B] E) k[A][B]<sup>2</sup>](https://d2lvgg3v3hfg70.cloudfront.net/TB6423/11eaa8f0_66fc_9d4b_93a6_7b0599911c04_TB6423_11.jpg) D (fast equilibrium)
Step 2: D + B E
Step 3: E + A C + B
If step 2 is the rate-determining step,then the rate of formation of C should equal:
D (fast equilibrium)
Step 2: D + B E
Step 3: E + A C + B
If step 2 is the rate-determining step,then the rate of formation of C should equal:
A) k[A]
B) k[A]2[B]
C) k[A]2[B]2
D) k[A][B]
E) k[A][B]2
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction aA ? Products, use the following choices -[A] is constant.
A) zero order in A
B) first order in A
C) second order in A
D) a, b, c
E) none of the above
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the reaction 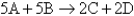 ,at a particular instant in time,the rate of the reaction is 0.0223 M/s.What is the rate of change of A?
,at a particular instant in time,the rate of the reaction is 0.0223 M/s.What is the rate of change of A?
A) -0.0223 M/s
B) 0.112 M/s
C) -0.112 M/s
D) -0.00446 M/s
E) 0.00446 M/s
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Showing 21 - 40 of 113
Related Exams