A) 1 < 2 < 3
B) 3 < 2 < 1
C) 2 < 3 < 1
D) 2 < 1 < 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you found a fossilized dinosaur bone, what could be done to accurately determine the age of the fossil?
A) Extract and sequence DNA from the bone.
B) Look at pieces of the bone under a microscope.
C) Analyze the isotopes of carbon in the fossil.
D) Compare the appearance of the bone to other fossilized bones.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Household ammonia, or ammonium hydroxide, is a mixture of ammonia (NH3) and water. What types of bonding interactions will occur between molecules of ammonia and water in a bottle of household ammonia?
A) polar covalent bonds between positively charged nitrogen atoms in ammonia and negatively charged oxygen atoms in water
B) polar covalent bonds between negatively charged nitrogen atoms in ammonia and positively charged hydrogen atoms in water
C) hydrogen bonds between positively charged nitrogen atoms in ammonia and negatively charged oxygen atoms in water
D) hydrogen bonds between negatively charged nitrogen atoms in ammonia and positively charged hydrogen atoms in water
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When full, the innermost electron shell of argon contains ________ electrons, and the outermost shell contains ________ electrons.
A) 2; 2
B) 2; 8
C) 4; 8
D) 8; 8
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water molecules stick to other water molecules because
A) water molecules are neutral, and neutral molecules are attracted to each other.
B) hydrogen bonds form between the hydrogen atoms of one water molecule and the oxygen atoms of other water molecules.
C) covalent bonds form between the hydrogen atoms of one water molecule and the oxygen atoms of other water molecules.
D) the oxygen atoms of adjacent water molecules are attracted to one another.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You've made a hot drink by dissolving a teaspoon of instant coffee and a teaspoon of sugar in a cup of hot water. Which of the following statements is true?
A) You've just prepared an aqueous solution.
B) The water is the solute portion of the drink.
C) The instant coffee is basic and the water is acidic so they mix well.
D) The instant coffee and sugar dissolve because they have no charged regions to repel the partial positive and partial negative regions of the water molecules.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons?
A) 6
B) 8
C) 12
D) 18
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following particles are found in the nucleus of an atom?
A) protons and neutrons
B) protons and electrons
C) neutrons and electrons
D) neutrons, electrons, and protons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
You want to design a controlled experiment to determine which tablet, PEPCID AC or Alka-Seltzer, neutralizes stomach acid the quickest. Which of the following experiments would be the best to perform?
A) Place a PEPCID AC tablet in a beaker of acid at pH 2 and an Alka-Seltzer tablet in a separate beaker of acid at pH 2 and check the pH in the beakers every 2 minutes.
B) Take a PEPCID AC tablet at 8:00 am on Monday and take an Alka-Seltzer tablet at 8:00 am on Tuesday and take note of how long it takes you to feel better.
C) Find a group of your friends and go out to a big dinner. Then give half of them a PEPCID AC tablet and give the other half an Alka-Seltzer tablet and tell them to write down how long it takes them to feel better.
D) Place a PEPCID AC tablet in a beaker of acid at pH 2 and an Alka-Seltzer tablet in a separate beaker of acid at pH 2 and record how long it takes for the tablets to dissolve.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What happens to an atom if the electrons in the outer shell are altered?
A) The atom becomes radioactive.
B) The atom becomes a different element.
C) The atom acquires different properties.
D) The atom loses a proton.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If you were able to chemically analyze your stomach fluids 30 minutes after taking two tablets, you would find
A) more hydrogen ions.
B) fewer hydrogen ions.
C) the same number of hydrogen ions.
D) that the pH in your stomach has decreased.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Below is the structure of leucine, an amino acid. What type of bond is the arrow pointing to? 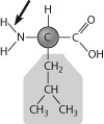
A) a nonpolar covalent bond
B) a polar covalent bond
C) an ionic bond
D) a hydrogen bond
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What change is occurring in this figure? 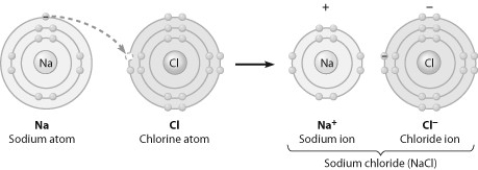
A) Chlorine is losing an electron.
B) Sodium is becoming negatively charged.
C) Sodium is filling its second electron shell.
D) Chlorine is filling its third electron shell.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 73 of 73
Related Exams