A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
The correct electron configuration for 17Cl is
A) 1s22s22p5
B) 1s22s22p63s2
C) 1s22s22p63s23p5
D) 1s22s22p63s23p64s23d5
Correct Answer

verified
C
Correct Answer
verified
Multiple Choice
Which of the following periodic table groups contains three or more nonmetallic elements?
A) IIIA
B) IVA
C) VA
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) The mass of an atom and the mass of its nucleus are essentially the same. (2) Two of the six electrons present in an atom of carbon are unpaired. (3) The element aluminum is located in the d area of the periodic table.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following characterizations for the element 52Te is correct?
A) both a representative element and a metal
B) electron configuration involves a partially filled s subshell
C) electron configuration involves a total of 52 electrons
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In which of the following pairs of elements are both members of the pair in the same period in the periodic table?
A) Ne and Mg
B) Ni and Pd
C) Ca and Se
D) K and Rb
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct atom that has a nuclear charge of +17
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is correct for 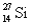 ?
?
A) contains more electrons than neutrons
B) contains no electrons
C) contains an equal number of neutrons and electrons
D) contains more neutrons than electrons
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the appropriate pair of atoms that contain the same number of subatomic particles.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following characterizations for the element 23V is correct?
A) a period 4 element
B) electron configuration ends in d3
C) an inner-transition element
D) more than one correct response
E) no correct response
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Two naturally occurring isotopes exist for the hypothetical element dippium. 25.0 percent of dippium atoms have a relative mass of 17.0 amu and 75.0 percent a relative mass of 21.3 amu. What is the atomic mass of dippium?
A) 18.1 amu
B) 19.1 amu
C) 20.2 amu
D) 38.3 amu
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Tin is element number 50. This means that all tin atoms have
A) an atomic number of 50
B) 50 neutrons in the nucleus
C) a total of 50 subatomic particles in the nucleus
D) more than one correct response
E) no correct response
Correct Answer

verified
A
Correct Answer
verified
Multiple Choice
Choose the appropriate electron configuration for an element with no unpaired electrons.
A) 1s22s22p63s23p6
B) 1s22s22p63s23p4
C) 1s22s22p63s1
D) 1s22s22p5
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) An electron configuration specifies subshell occupancy for electrons. (2) The atomic number is double the mass number for atoms that contain an equal number of protons and neutrons. (3) All occupied orbitals are completely filled in atoms of the element neon, which contains a total of 12 electrons.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose an appropriate pair of elements that have similar chemical properties.
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the appropriate pair of atoms that contain the same number of neutrons.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Characterize EACH of the following statements as being TRUE or FALSE and then indicate the collective true-false status using the choices provided. (1) All of the major minerals in the human body are metals. (2) Iron is the most abundant transition metal in the human body. (3) Deuterium is an isotope of hydrogen in which two protons are present.
A) All three statements are true.
B) Two of the three statements are true.
C) Only one of the statements is true.
D) None of the statements is true.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct atom that has an equal number of protons, neutrons, and electrons.
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose an appropriate pair of elements that are in the same group of the periodic table.
A) (37Rb and 38Sr)
B) (7N and 82Pb)
C) (9F and 17Cl)
D) (42Mo and 75Re)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements is both a metal and a representative element?
A) (9F)
B) (50Sn)
C) (79Au)
D) (92U)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 70
Related Exams