A) 3.4 103 years
B) 4.3 103 years
C) 4.9 103 years
D) 1.9 103 years
E) 3.0 103 years
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The U-238 nucleus decays to form Pb-206 by and decays. -Calculate the number of decays.
A) 2
B) 4
C) 6
D) 8
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The Br-82 nucleus has a half-life of about 1.0 103 minutes. If you needed at least 1.6 g of Br-82 and had ordered 29 g of NaBr (assuming all of the Br in the NaBr was Br-82) , how many days could you wait for delivery?
A) 3.2 days
B) 1.2 days
C) 2.9 days
D) 2.6 days
E) 3.8 days
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radioactive tracers are useful in studying very low concentrations of chemical species. A chemist has a sample of HgI2 in which part of the iodine is the radioactive nuclide of mass 131, so that the count rate is 5.0 1011 counts per minute per mole of I. The solid mercuric iodide is placed in water and allowed to come to equilibrium. Then 100 mL of the solution is withdrawn, and its radioactivity is measured and found to give 22 counts per minute. What is the molar concentration of iodide ion in the solution?
A) 1.1 10-11
B) 1.1 10-9
C) 1.1 10-10
D) 4.4 10-10
E) 4.4 10-11
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of 90Sr is 28 years. How long will it take for a given sample of 90Sr to be 86% decomposed?
A) 3.4 101 years
B) 2.6 years
C) 3.3 102 years
D) 6.1 years
E) 7.9 101 years
Correct Answer

verified
Correct Answer
verified
Multiple Choice
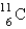 is an unstable isotope. Which radioactive decay would be expected?
is an unstable isotope. Which radioactive decay would be expected?
A) ![]()
B) ()
C) fission
D) ()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the U-235 nucleus is struck with a neutron, the Ce-144 and Sr-90 nuclei are produced, along with some neutrons and beta particles. -How many neutrons are emitted?
A) 5
B) 6
C) 2
D) 3
E) 4
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The ratio of the atomic radius to the nuclear radius is approximately
A) 105
B) 10-5
C) 10-15
D) 102
E) 1015
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which reaction will produce an isotope of the parent nuclide?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the most likely decay for the Co-62 nucleus?
A) positron emission
B) (-ray emission)
C) ( decay)
D) ( decay)
E) proton emission
Correct Answer

verified
Correct Answer
verified
Multiple Choice
When the Pd-106 nucleus is struck with an alpha particle, a proton is produced along with a new element. What is this new element?
A) Cd-109
B) Ag-108
C) Ag-109
D) Cd-112
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The questions below refer to the following:
Iron-56, 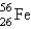 , has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 10-13 J)
-Determine the amount of energy needed to "decompose" 1 mol of iron-56 nuclei.
, has a binding energy per nucleon of 8.79 MeV. (1 MeV = 1.60 10-13 J)
-Determine the amount of energy needed to "decompose" 1 mol of iron-56 nuclei.
A) 4.74 1013 J
B) 3.47 1011 J
C) 8.90 1011 J
D) 1.13 1014 J
E) 7.75 1013 J
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The U-238 nucleus decays to form Pb-206 by and decays. -Calculate the number of decays.
A) 4
B) 2
C) 8
D) 6
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is a product of decay of 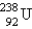 ?
?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Radioactive elements decay via first-order kinetics. Consider a certain type of nucleus that has a rate constant of 1.6 10-2 h-1. A sample contains 7.9 108 radioactive nuclides. Calculate the time required for 63% of the nuclides to decompose.
A) 1.3 101 h
B) 1.3 10-1 h
C) 2.7 101 h
D) 2.9 101 h
E) 6.2 101 h
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The stable nuclide 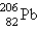 is formed from
is formed from 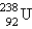 by a long series of and decays. Which of the following nuclides could not be involved in this decay series?
by a long series of and decays. Which of the following nuclides could not be involved in this decay series?
A) Po-221
B) Pu-239
C) Tl-210
D) Ra-226
E) Pa-234
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following balanced equations is labeled incorrectly?
A) beta production: ![]()
B) bombardment: ![]()
C) alpha production: ![]()
D) fusion: ![]()
E) fusion: ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life for electron capture for 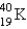 is 1.3 billion years. What will be the
is 1.3 billion years. What will be the 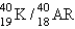 ratio in a rock that is 4.5 billion years old?
ratio in a rock that is 4.5 billion years old?
A) 0.10
B) 0.091
C) 10.
D) 11.
E) 0.36
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If more than one neutron from each fission event causes another fission event, the fission situation is described as
A) supercritical.
B) critical.
C) moderated.
D) subcritical.
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the most likely decay for the Fe-53 nucleus?
A) (-ray emission)
B) ( decay)
C) ( decay)
D) positron emission
E) two of these
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 68
Related Exams