A) 120°.
B) 109°.
C) 150°.
D) 90°.
E) 360°.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following molecules. I. BF3 II. CHBr3 (C is the central atom.) III. Br2 IV. XeCl2 V.CO VI. SF4 Select the molecule(s) that fit the given statement. These molecules have a zero net dipole moment.
A) III, V
B) III, IV, V
C) I, III, IV
D) I, III, IV, VI
E) none of them
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for XeCl4.
A) square planar
B) octahedral
C) tetrahedral
D) pyramidal
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for NH3.
A) linear
B) tetrahedral
C) pyramidal
D) bent
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the compound crotonaldehyde, whose skeleton is 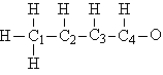 -How many electrons must be shown in the Lewis structure of this molecule?
-How many electrons must be shown in the Lewis structure of this molecule?
A) 32
B) 28
C) 18
D) 24
E) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for PF3.
A) pyramidal
B) square planar
C) tetrahedral
D) octahedral
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Use the following electronegativity values to answer the question: C 2.5 Cl 3.2 H 2.2 N 3.0 O 3.4 This molecule contains a carbon atom with trigonal planar geometry.
A) C2H6
B) CO2
C) CH3Cl
D) CH3CHO
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following has the largest radius?
A) Na+
B) Ne
C) O2-
D) Mg2+
E) F-
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is polar?
A) NBr3
B) XeF4
C) SBr6
D) KrF2
E) BBr3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the shape of the ICl5 molecule?
A) square pyramid
B) octahedral
C) trigonal bipyramid
D) see-saw
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Select the correct molecular structure for NO3-.
A) square planar
B) pyramidal
C) octahedral
D) tetrahedral
E) none of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many lone pairs of electrons are around the central atom?
A) 1
B) 4
C) 0
D) 2
E) 3
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The first electron affinity value for oxygen is _______ and the second electron affinity value is ________.
A) favorable (exothermic) , favorable (exothermic)
B) More information is needed.
C) favorable (exothermic) , unfavorable (endothermic)
D) unfavorable (endothermic) , favorable (exothermic)
E) unfavorable (endothermic) , unfavorable (endothermic)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following molecules has a dipole moment?
A) SCl6
B) CO2
C) OF2
D) BH3
E) None of these has a dipole moment.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which species must be polar?
A) CO ![]()
B) CH4
C) C2H4
D) CO2
E) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following elements forms the most ionic bond with chlorine?
A) Ar
B) Ga
C) P
D) I
E) Cs
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the compound with the most ionic bond.
A) LiF
B) RbBr
C) KBr
D) KF
E) NaBr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What type of structure does the XeOF2 molecule have?
A) trigonal planar
B) octahedral
C) pyramidal
D) T-shaped
E) tetrahedral
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following bonds would be the least polar and yet still be considered polar covalent?
A) Mg-S
B) Ga-S
C) B-S
D) P-S
E) S-S
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following molecules. I. BF3 II. CHBr3 (C is the central atom.) III. Br2 IV. XeCl2 V. CO VI. SF4 Select the molecule(s) that fit the given statement. These molecules violate the octet rule.
A) III, V, VI
B) I, II, IV, VI
C) I, IV, VI
D) I, II, IV
E) I, III, IV, VI
Correct Answer

verified
Correct Answer
verified
Showing 101 - 120 of 135
Related Exams