A) Medicinal applications
B) Food preservation
C) Studies of piston wear
D) Monitoring the progress of certain oils through pipelines
E) All of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following best describes a natural radioactive decay series?
A) Change of one element to another by nuclear bombardment
B) A chain reaction,in which a nuclear product of one reaction becomes a nuclear reactant in the next step
C) The combination of two small nuclei to form a larger nucleus
D) A list of a series of reactions that illustrate how a radioactive element disintegrates into a stable isotope
E) A nuclear equation for a natural radioactive decay,showing the alpha and beta particles written as a series rather than with coefficients
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is or are false? (i) Radioactivity first appeared on earth in the late 1800s. (ii) Atoms cannot be changed from one element to another. (iii) If a sample of a substance is radioactive,the sample remains radioactive forever.
A) i only
B) i and ii
C) i and iii
D) ii and iii
E) i,ii,and iii
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The half-life of 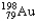 is 2.7 days.If you begin with 25 grams of
is 2.7 days.If you begin with 25 grams of 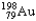 ,how many grams will you have 10.8 days later?
,how many grams will you have 10.8 days later?
A) 1.6 g
B) 2.3 g
C) 6.3 g
D) 9.3 g
E) 0.32 g
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What distinguishes natural radioactivity from induced radioactivity produced by bombardment reactions?
A) Gamma rays are not emitted in natural radioactivity
B) Gamma rays are not emitted in induced radioactivity
C) Induced radioactivity produces radioactive elements not found in nature
D) Induced radioactivity is still a theoretical concept; natural radioactivity actually occurs
E) When naturally radioactive substances decay,they emit natural radiation,but when induced radioactive substances decay,they emit artificial radiation
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following units is most commonly used to express radiation exposure?
A) rem
B) R
C) J
D) rad
E) All can be used to measure radiation exposure.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
An ancient tree is found which has a carbon-14 disintegration rate of 11.3 units.If the disintegration rate of a living tree is 14.0 units,calculate the age of the ancient tree.t1/2 = 5.73 * 103 years for carbon-14 and 0.807 of the sample remaining corresponds to 0.309 half-life.
A) 1.77 * 103 yr
B) 4.62 * 103 yr
C) 5.73 *103 yr
D) 1.85 * 104 yr
E) 7.10 * 104 yr
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following represents the correct nuclear equation for the alpha decay of 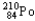 ?
?
A) ![]()
![]()
+ ![]()
B) ![]() +
+ ![]()
![]()
C) ![]()
![]() +
+ ![]()
D) ![]() +
+ ![]()
![]()
E) ![]()
![]() +
+ ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct nuclear symbol for the missing species to complete the following nuclear bombardment equation: 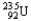 + ?
+ ? 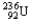
A) ![]()
B) ![]()
C) ![]()
D) ![]()
E) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is or are reasons why the building and continued use of nuclear power plants faces some opposition in the United States? (i) The threat of an accident that might release large amounts of radiation. (ii) Disposal of the radioactive wastes that are generated by nuclear reactors. (iii) Fear that an irresponsible government may use nuclear fuel to manufacture nuclear weapons.
A) iii only
B) i and ii
C) i and iii
D) ii and iii
E) i,ii,and iii
Correct Answer

verified
Correct Answer
verified
Showing 41 - 50 of 50
Related Exams