A) The reverse reaction rate is at a maximum
B) The concentration of A must equal the concentration of B
C) The concentration of A must equal one-half the concentration of B
D) The forward reaction rate is equal to the reverse reaction rate
E) The forward reaction rate is equal to the equilibrium reaction rate
G) All of the above
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
Which of the following conditions is not found in a system that is at equilibrium?
A) Reactants and products are being converted into one another continually
B) The forward rate of reaction is equal to the rate of the reverse reaction
C) The concentration of reactants is equal to the concentration of the products
D) The system is closed and isolated from its surroundings
E) The reaction can be represented by an equation with a double arrow
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following correctly expresses Ka for the weak acid HF(aq) ?
A) Ka = [HF]
B) Ka = ![]()
C) Ka = ![]()
D) Ka = [H+][F-]
E) Ka = ![]()
G) A) and B)
Correct Answer

verified
B
Correct Answer
verified
Multiple Choice
Consider the following reaction coordinate diagram. 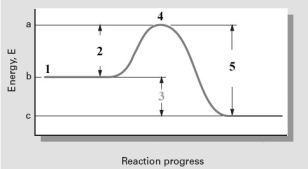 Which numbered point represents the transition state of the reaction?
Which numbered point represents the transition state of the reaction?
A) 1
B) 2
C) 3
D) 4
E) 5
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How does a catalyst alter the rate of a reaction?
A) Catalysts replace part of the reactants
B) Catalysts provide the activation energy needed
C) A catalyst interferes with the attainment of chemical equilibrium
D) Catalysts provide more heat to the reaction,and the increased temperature leads to an increased reaction rate
E) Catalysts provide a reaction pathway with a lower energy of activation
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the best description of the activation energy for the forward reaction for the reaction A2 + B2  2 AB?
2 AB?
A) The difference between the energy of the activated complex and the reactant energies
B) The difference between the product energies and the energy of the activated complex
C) The difference between the product energies and the reactant energies
D) The sum of the energy of the activated complex and the reactant energies
E) The sum of the energy of the activated complex and the product energies
G) A) and B)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What will happen if the temperature is increased after equilibrium has been established in the reaction shown? 2 Cl2(g) + 2 H2O(g) + 113 kJ  4 HCl(g) + O2(g)
4 HCl(g) + O2(g)
A) The position of equilibrium shifts to the left
B) More HCl will form
C) More Cl2 will form
D) The partial pressure of oxygen will decrease
E) The concentration of H2O will increase
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements does not correctly describe the character of a chemical equilibrium?
A) As a system comes to equilibrium,the chemical reactions come to a stop
B) In a liquid-gas equilibrium,the number of moles of the gas does not necessarily equal the number of moles of the liquid
C) In a solution equilibrium,the number of moles of the solvent does not necessarily equal the number of moles of the liquid
D) All of the statements above correctly describe a chemical equilibrium
E) None of the statements above correctly describes a chemical equilibrium
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the following reaction coordinate diagram. 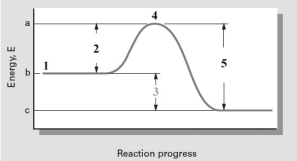 Based on this diagram,which of the following is correct?
Based on this diagram,which of the following is correct?
A) 2 represents E and the reaction is exothermic.
B) 2 represents E and the reaction is endothermic
C) 3 represents E and the reaction is endothermic
D) 3 represents E and the reaction is exothermic
E) 5 represents E and the reaction is exothermic
G) A) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the position of equilibrium shifts to the left,what will happen?
A) The concentration of products will increase
B) The concentration of reactants will increase
C) The temperature will rise
D) The temperature will decrease
E) The concentrations of both products and reactants will increase
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What will happen to the position of equilibrium if the volume of the system is decreased in the reaction shown? N2(g) + 3 H2  2 NH3(g)
2 NH3(g)
A) It will shift to the left
B) There will be no shift in the position of equilibrium
C) The position will shift left and then return to its original place
D) It will shift to the right
E) It will shift so that the system volume is increased again
G) All of the above
Correct Answer

verified
D
Correct Answer
verified
Multiple Choice
What will happen if the temperature is decreased after equilibrium has been established in the reaction below? 2 C2H6(g) + 7 O2(g)  4 CO2(g) + 6 H2O(g) + 2855 kJ
4 CO2(g) + 6 H2O(g) + 2855 kJ
A) There will be no change in the system
B) The concentration of ethane will increase
C) The partial pressure of oxygen will increase
D) The number of water molecules will decrease
E) More carbon dioxide will form
G) A) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Choose the correct equilibrium constant expression for the equilibrium CO(g) + Cl2(g)  COCl2(g) .
COCl2(g) .
A) K = ![]()
B) K = ![]()
C) K = ![]()
D) K = ![]()
E) K = ![]()
G) B) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What kind of molecular collisions will not lead to the formation of product in a reaction?
A) Only those without sufficient kinetic energy
B) Only those with the wrong orientation,regardless of the kinetic energy
C) Collisions between molecules that have more energy than the minimum required
D) Those without sufficient kinetic energy and with the wrong orientation
E) Those with effective collisions
G) B) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is/are incorrect? (i) Undissolved sugar in contact with a saturated sugar solution in an open beaker cannot reach equilibrium (ii) A liquid and its vapor enclosed in two one-liter containers connected by an open glass tube cannot reach equilibrium (iii) A solution of a weak acid,hydronium ion,and the conjugate base of the weak acid cannot reach equilibrium
A) i only
B) ii only
C) i and ii
D) ii and iii
E) i,ii,and iii
G) B) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the primary reason for a higher reaction rate at higher temperatures?
A) Molecules move faster at higher temperatures,producing more violent collisions
B) Electrons occupy higher principal energy levels and are therefore more reactive
C) There is a larger fraction of molecules with sufficient energy to react
D) Molecules increase in size with increasing temperature,providing more surface area for potential collisions
E) The higher temperature creates a lower activation energy,so more molecules can pass over the potential energy barrier
G) A) and C)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is/are correct? (i) The forward reaction in an equilibrium reaction is exactly the reverse of the reverse reaction (ii) A dynamic equilibrium is established when two molecules collide (iii) As reactant concentrations in a chemical equilibrium reaction decrease,the forward reaction rate declines
A) ii only
B) i and ii
C) i and iii
D) ii and iii
E) i,ii,and iii
G) D) and E)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements is/are correct? (i) If an equilibrium constant is very large,the reverse reaction is favored (ii) If an equilibrium constant is very small,the forward reaction is favored (iii) If an equilibrium constant is neither large nor small,appreciable quantities of all species are present at equilibrium
A) iii only
B) i and ii
C) ii and iii
D) All statements are incorrect
E) All statements are correct
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the particulate level representation of a reaction of the form: 
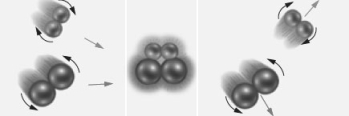 Based on just this diagram,which of the following does not correctly describes this reaction?
Based on just this diagram,which of the following does not correctly describes this reaction?
A) The collision was ineffective.
B) Product did not form.
C) The orientation of the reactants to with respect to each other was wrong.
D) The molecules had too much potential energy.
E) All of the above do describe this reaction.
G) None of the above
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If a copper(II) nitrate solution is added to a sodium sulfide solution the following equilibrium will be attained: Cu2+(aq) + S2-(aq)  CuS(s) .For this equilibrium,K = 6.3*1047.Choose the correct equilibrium constant expression and the correct direction favored at equilibrium.
CuS(s) .For this equilibrium,K = 6.3*1047.Choose the correct equilibrium constant expression and the correct direction favored at equilibrium.
A) K = ![]() forward
forward
B) K = ![]() reverse
reverse
C) K = ![]() forward
forward
D) K = ![]() reverse
reverse
E) K = ![]() appreciable quantities of all species are present at equilibrium
appreciable quantities of all species are present at equilibrium
G) C) and D)
Correct Answer

verified
Correct Answer
verified
Showing 1 - 20 of 44
Related Exams