Correct Answer

verified
Correct Answer
verified
Multiple Choice
_____ are the subunits of nucleic acids, and _____ are the subunits of proteins.
A) Nucleotides; amino acids
B) Bases; polypeptides
C) Polypeptides; sugars
D) Amino acids; nucleic bases
E) Nucleoli; amino acids
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following statements about water is CORRECT?
A) Ice is less dense than liquid water.
B) Ice forms on top and sinks to the bottom of lakes and rivers.
C) Ice is more dense than liquid water.
D) Bodies of water freeze from the bottom up.
E) Water molecules in ice demonstrate a disorganized, non-lattice arrangement.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Samples of three different triacylglycerols were tested to determine the melting point of each one. The results of the tests are shown in the graph.
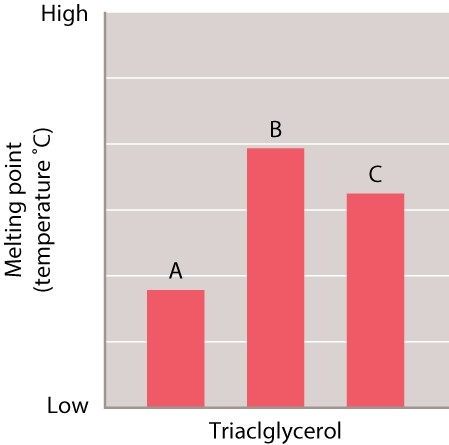 The length of the fatty acids in A, B, and C is the same. Which of the three fatty acids is likely to have the MOST saturated fatty acids?
The length of the fatty acids in A, B, and C is the same. Which of the three fatty acids is likely to have the MOST saturated fatty acids?
A) A
B) B
C) C
D) There is no way of knowing based on the information available.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following ranks the elements carbon, sodium, calcium, and iodine in order of decreasing number of protons?
A) CNaCaI
B) ICaNaC
C) ICCaNa
D) CCaNaI
Correct Answer

verified
Correct Answer
verified
Multiple Choice
How many hydrogen atoms are present in a ring of six carbon atoms held together by alternating single and double bonds?
A) 6
B) 8
C) 10
D) 12
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Rank the elements carbon, phosphorus, calcium, and iodine in order of decreasing number of energy shells/levels.
A) CPCaI
B) ICaPC
C) IPCCa
D) PCCaI
Correct Answer

verified
Correct Answer
verified
Short Answer
Rank the elements carbon, sodium, calcium, and iodine in order of greatest abundance in living organisms.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Water readily dissolves compounds that are referred to as:
A) hydrophobic.
B) solvent.
C) nonpolar.
D) hydrophilic.
E) aqueous.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Refer to the periodic table, and decide which of the following molecules is held together by polar covalent bonds. 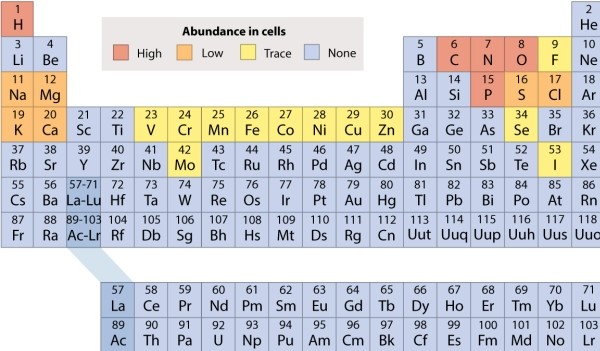
A) NH3
B) CO2
C) KCl
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following statements about carbon is CORRECT?
A) Each of carbon's three valence electrons shares a bond with another atom.
B) Carbon-carbon single bonds allow for free rotation around the bond; carbon-carbon double bonds do not allow for free rotation around the bond.
C) The spatial orientation of carbon's bonds results in a carbon atom resting in the center of a three-dimensional structure referred to as a hexahedron.
D) Carbon is not only the most abundant element on Earth, but it is believed to be the most abundant element in the universe.
E) Single bonds between carbon atoms are typically shorter than double bonds between carbon atoms.
Correct Answer

verified
Correct Answer
verified
Matching
Complete the matching exercise below by choosing the CORRECT strength of each bond type in aqueous solution. Responses may be used once, more than once, or not at all.
Correct Answer
Multiple Choice
Which of the following organic molecules is commonly used for energy storage?
A) proteins and nucleic acids
B) lipids and carbohydrates
C) nucleic acids and lipids
D) carbohydrates, nucleic acids, and lipids
E) proteins, carbohydrates, and lipids
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following elements is found in every organic molecule?
A) carbon
B) phosphorus
C) nitrogen
D) oxygen
E) sulfur
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Some species of insects are able to walk across liquid water because:
A) of the high surface tension of water due to the hydrophobic effect.
B) insects have a low center of gravity.
C) of the higher density of liquid water compared to solid water.
D) water has high surface tension due to ionic bonding.
E) of the high surface tension of water due to its cohesion.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The negatively charged components of atoms are referred to as:
A) protons.
B) electrons.
C) anions.
D) neutrons.
E) cations.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Because of hydrogen bonding, water is uniquely suited for its central role in life. Many hydrophilic molecules interact freely with water, but a number of hydrophobic molecules are important for life, too. How does the interaction between water and hydrophobic molecules help to organize biological systems?
A) Because cells are not pure water (they have many substances dissolved within them) , the hydrophilic/hydrophobic effect has a limited role in biological organization.
B) The ionic bonds between water molecules cause hydrophobic molecules to associate with each other and not with water molecules.
C) Because water molecules preferentially associate with each other, they force hydrophobic molecules to associate with each other and not with water molecules.
D) None of the other answer options is correct.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Imagine you were there when Stanley Miller performed his experiment to produce the building blocks of life. If Miller originally identified 5 different amino acids, how many polypeptides that are 10 amino acids long could be made from just these 5 amino acids?
A) 5 × 10 = 50
B) 105 = 100,000
C) 510 = 9,765, 625
D) The answer cannot be determined from the information provided.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which one of the following MOST accurately describes the ratio of oxygen to carbon to hydrogen in a simple 6-carbon sugar such as glucose?
A) 1:2:1
B) 1:1:2
C) 2:1:1
D) 1:2:3
E) 1:3:2
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Samples of three different triacylglycerols were tested to determine the melting point of each one. The results of the tests are shown in the graph. 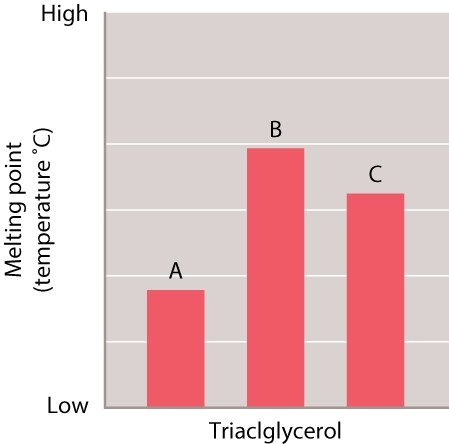 The length of the fatty acids in A, B, and C is the same. Which of the three triacylgcerols is likely to have the FEWEST number double bonds in the fatty acids?
The length of the fatty acids in A, B, and C is the same. Which of the three triacylgcerols is likely to have the FEWEST number double bonds in the fatty acids?
A) A
B) B
C) C
D) There is no way of knowing based on the information available.
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 232
Related Exams