A) The rate increases as equilibrium is approached.
B) The rate decreases as equilibrium is approached.
C) The rate remains constant.
D) The rate slows down, then increases, as equilibrium is approached.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For an endothermic reaction, lowering the temperature of the reaction will ________.
A) increase the amount of products
B) decrease the amount of reactants
C) decrease the amount of products
D) both A and B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the reaction vessel is expanded, this will ________. CH₄ (g) + 2O₂ (g)  CO₂ (g) + 2H₂O (g)
CO₂ (g) + 2H₂O (g)
A) shift the reaction to the right
B) shift the reaction to the left
C) have no effect
D) cannot be determined, since the temperature is unknown
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Removing ammonia as soon as it is formed will ________. N₂ (g) + 3H₂ (g)  2NH₃ (g)
2NH₃ (g)
A) shift the reaction to the right
B) shift the reaction to the left
C) have no effect
D) cannot be determined, since the temperature is unknown
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Removing product will ________. 2SO₂ (g) + O₂ (g)  2SO₃ (g)
2SO₃ (g)
A) shift the reaction to the right
B) shift the reaction to the left
C) have no effect
D) cannot be determined, since the temperature is unknown
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Decreasing the concentration of ammonia gas will ________. 2NH₃ (g)  N₂ (g) + 3H₂ (g)
N₂ (g) + 3H₂ (g)
A) shift the reaction to the right
B) shift the reaction to the left
C) have no effect
D) cannot be determined, since the temperature is unknown
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Calculate the equilibrium concentration of N₂ for the following reaction. 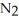 +
+ 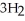

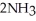 The equilibrium constant Keq is 0.055 and the concentrations of
The equilibrium constant Keq is 0.055 and the concentrations of 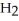 and
and 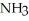 are 1.20 M and 0.225 M, respectively.
are 1.20 M and 0.225 M, respectively.
A) 0.0915 M
B) 0.533 M
C) 0.915 M
D) 3.41 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Match each reaction and its equilibrium concentrations with its corresponding equilibrium constant value.
-N?O5 (g) ![Match each reaction and its equilibrium concentrations with its corresponding equilibrium constant value. -N?O<sub>5</sub> (g) 2 O? (g) + [N?O] = 0.50 M, [O?] = 1.0 M, [N?O<sub>5</sub>] = 0.10 M A) 25 B) 12.3 C) 5.0](https://d2lvgg3v3hfg70.cloudfront.net/TB6848/11eab3b7_63f9_4955_be6e_f79dad4ae41c_TB6848_11.jpg) 2 O? (g) +
2 O? (g) + ![Match each reaction and its equilibrium concentrations with its corresponding equilibrium constant value. -N?O<sub>5</sub> (g) 2 O? (g) + [N?O] = 0.50 M, [O?] = 1.0 M, [N?O<sub>5</sub>] = 0.10 M A) 25 B) 12.3 C) 5.0](https://d2lvgg3v3hfg70.cloudfront.net/TB6848/11eab3b7_63f9_7066_be6e_cfd519dca1fd_TB6848_11.jpg) [N?O] = 0.50 M, [O?] = 1.0 M, [N?O5] = 0.10 M
[N?O] = 0.50 M, [O?] = 1.0 M, [N?O5] = 0.10 M
A) 25
B) 12.3
C) 5.0
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In an exothermic reaction, adding heat will ________.
A) increase the amount of products
B) decrease the amount of reactants
C) decrease the amount of products
D) both A and B
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Consider the reaction: C (s) + CO? (g)  2 CO (g) (endothermic) .
Match each equilibrium change with its corresponding consequence.
-decreasing the pressure
2 CO (g) (endothermic) .
Match each equilibrium change with its corresponding consequence.
-decreasing the pressure
A) Equilibrium is shifted to the right.
B) No effect on the equilibrium.
C) Equilibrium is shifted to the left.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
If the solubility of calcium fluoride is 2.0 × 10-4 M, the solubility product value is ________.
A) 1.6 × 10-12
B) 3.2 × 10-11
C) 4.0 × 10-8
D) 8.0 × 10-12
Correct Answer

verified
Correct Answer
verified
True/False
Removing ozone as soon as it is formed will shift the equilibrium to the right. 3O₂ (g) ⇔ 2O₃ (g)
Correct Answer

verified
Correct Answer
verified
Essay
Write an expression for the equilibrium constant (Keq) for the following reaction.
HCl (g) + H₂O (l)  H₃O+ (aq)+ Cl- (aq)
H₃O+ (aq)+ Cl- (aq)
Correct Answer

verified
Keq = [  ]...
]...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
Predict the effect of increasing pressure in each of the reactions by choosing the appropriate direction shift.
-2 SO₃ (g) + 2 CL₂ (g) 
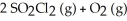
A) no effect
B) right (→)
C) left (←)
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The solubility of SrF₂ in water (given Ksp = 2.6 × 10-9 ) is ________.
A) 4.3 × 10-4 M
B) 1.2 × 10-4 M
C) 7.6 × 10-5 M
D) 8.7 × 10-4 M
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For an exothermic reaction, lowering the temperature of the reaction will ________.
A) increase the amount of products
B) decrease the amount of reactants
C) decrease the amount of products
D) both A and B
Correct Answer

verified
Correct Answer
verified
True/False
Adding a catalyst to any reaction will always change the value of the equilibrium constant.
Correct Answer

verified
Correct Answer
verified
True/False
At equilibrium, the amount of product present is always greater than the amount of reactant present.
Correct Answer

verified
Correct Answer
verified
True/False
The double arrow in a chemical reaction indicates that the equilibrium is established since the rate of the forward reaction is equal to the rate of the reverse reaction.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The equilibrium concentrations for the reaction at 300 K are [CL₂] = 0.75 M, [PCL₃] = 0.45 M, and [PCl5] = 0.73 M. The value of Keq is ________. CL₂ (g) + PCL₃ (g) ![The equilibrium concentrations for the reaction at 300 K are [CL₂] = 0.75 M, [PCL₃] = 0.45 M, and [PCl5] = 0.73 M. The value of Keq is ________. CL₂ (g) + PCL₃ (g) PCl5 (g) A) 2.2 B) 0.15 C) 0.048 D) 4.7](https://d2lvgg3v3hfg70.cloudfront.net/TB6848/11eab3b7_63f0_9739_be6e_cf42dcc266f2_TB6848_11.jpg) PCl5 (g)
PCl5 (g)
A) 2.2
B) 0.15
C) 0.048
D) 4.7
Correct Answer

verified
Correct Answer
verified
Showing 61 - 80 of 135
Related Exams