A) A local maximum on the energy diagram.
B) A point on the reaction pathway that has a discrete lifetime.
C) A point half-way between the starting materials and products.
D) The highest energy compound on an energy diagram.
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following cannot be an electrophile?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is a transition state?
A) An isolable intermediate in a reaction.
B) The starting materials of the reaction.
C) A local maximum on the energy diagram.
D) A low-energy point between the starting materials and the product.
Correct Answer

verified
Correct Answer
verified
Short Answer
What pattern of curved arrow pushing is the second step of this reaction? 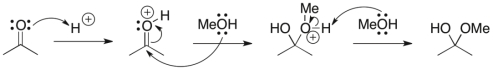
Correct Answer

verified
Correct Answer
verified
Short Answer
Given the following rate law, what is the order of the reaction with respect to MeI? 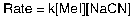
Correct Answer

verified
Correct Answer
verified
Essay
Draw an energy diagram for an endothermic reaction with two steps.
Correct Answer

verified
Correct Answer
verified
Short Answer
Will the following cation undergo rearrangement? 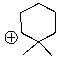
Correct Answer

verified
Correct Answer
verified
Short Answer
Predict the sign of S of the following reaction. 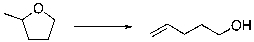
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the nucleophilic site in the reactants of the following reaction? 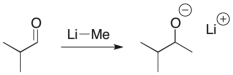
A) C of the carbonyl
B) O
C) Li
D) Me of Li-Me
Correct Answer

verified
Correct Answer
verified
Essay
Melphalan, a drug used in chemotherapy, reacts with itself in the body before binding with its target, as illustrated in the mechanism below. Which two patterns of arrow pushing are seen in this reaction? 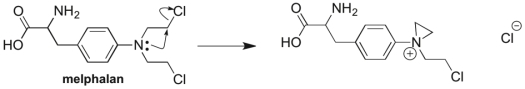
Correct Answer

verified
Nucleophil...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Melphalan, a drug used in chemotherapy, reacts with itself before binding with its target in the body, as illustrated below. Draw in the curved arrows that account for this transformation. 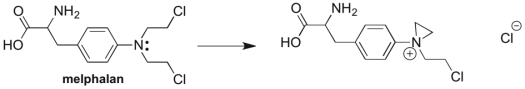
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction step, indicate which pattern of arrow pushing it represents. 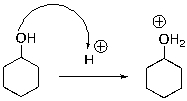
A) Proton transfer
B) Loss of leaving group
C) Nucleophilic attack
D) Rearrangement
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following reaction step, indicate which pattern of arrow pushing it represents. 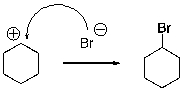
A) Proton transfer
B) Loss of leaving group
C) Nucleophilic attack
D) Rearrangement
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the electrophilic site in the following molecule. 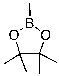
A) C
B) O
C) B
D) No electrophilic site
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following cations is most likely to undergo rearrangement?
A) ![]()
B) ![]()
C) ![]()
D) ![]()
Correct Answer

verified
Correct Answer
verified
Essay
Identify the nucleophilic site in the following molecule. 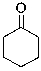
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is the electrophilic site in the reactants of the following reaction? 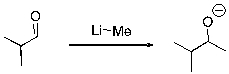
A) C of the carbonyl
B) O
C) Li
D) Me
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Predict the sign of G for an exothermic reaction with an increase in entropy.
A) positive
B) negative
C) no change
D) cannot predict without additional information
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Identify the electrophilic site in the following molecule. 
A) H
B) C of carbonyl
C) C other than carbonyl
D) O of carbonyl
Correct Answer

verified
Correct Answer
verified
Essay
Draw the mechanism and most likely product for the following cation rearrangement. 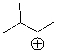
Correct Answer

verified
Correct Answer
verified
Showing 41 - 60 of 110
Related Exams