A) a negatively charged ion
B) a positively charged ion
C) a sodium atom
D) Both B & C
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the Keq for the following reaction? 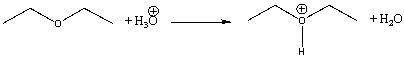
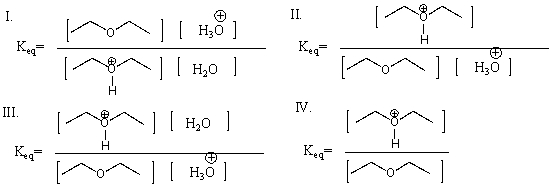
A) I
B) II
C) III
D) IV
E) Both II & III
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid-base reaction, predict which side the equilibrium is favored. 
A) favor right side
B) favor left side
C) neither
Correct Answer

verified
Correct Answer
verified
Multiple Choice
In a Bronsted-Lowry acid-base reaction the product(s) is (are) a _______.
A) Lewis acid
B) conjugate acid only
C) Lewis acid-base pair
D) conjugate acid-conjugate base pair
E) None of these
Correct Answer

verified
Correct Answer
verified
Essay
Predict the products for the following acid-base reaction. 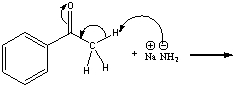
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following is not a Bronsted-Lowry acid?
A) CH3OH
B) CH3CH3
C) CH3NH2
D) CH3SH
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is most basic? 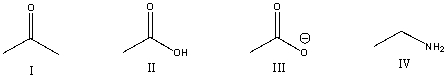
A) I
B) II
C) III
D) IV
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
Which of the following compounds is most acidic? 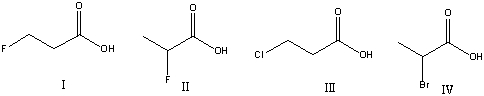
A) I
B) II
C) III
D) IV
Correct Answer

verified
Correct Answer
verified
Essay
Provide a definition of a Brønsted-Lowry acid.
Correct Answer

verified
A Brønsted...View Answer
Show Answer
Correct Answer
verified
View Answer
Multiple Choice
A loss of proton from a Brønsted-Lowry acid results in a ___.
A) Lewis acid
B) conjugate acid
C) conjugate base
D) conjugate acid-base pair
E) None of these
Correct Answer

verified
Correct Answer
verified
Multiple Choice
For the following acid-base reaction, predict which side the equilibrium is favored. 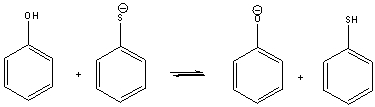
A) favor right side
B) favor left side
C) neither
Correct Answer

verified
Correct Answer
verified
Essay
Provide a curved arrow mechanism for the following acid-base reaction. 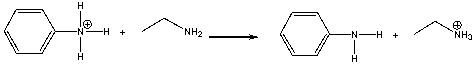
Correct Answer

verified
Correct Answer
verified
Essay
Provide a curved arrow mechanism for the following acid-base reaction. 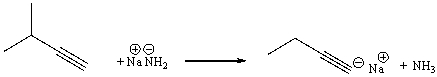
Correct Answer

verified
Correct Answer
verified
Essay
Determine if H2O is a suitable reagent to protonate the following compound. Explain why. Draw the complete reaction, including the curved arrow mechanism. 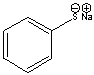
Correct Answer

verified
No.  Sulfur is a larger atom compared to...
Sulfur is a larger atom compared to...View Answer
Show Answer
Correct Answer
verified
View Answer
Essay
Identify the acid and the base and provide a curved arrow mechanism for the following reaction. 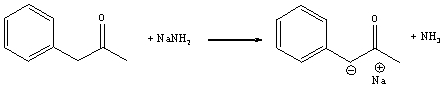
Correct Answer

verified
Correct Answer
verified
Multiple Choice
BF3, is best classified as a ____.
A) Brønsted-Lowry acid
B) Lewis acid
C) Brønsted-Lowry base
D) Lewis base
E) Both A & D
Correct Answer

verified
Correct Answer
verified
Multiple Choice
The following reaction mechanism contains error. Which of the following statements correctly describes the curved arrows consistent with the reaction? 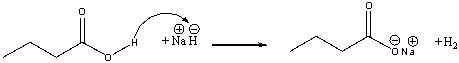
A) The curved arrow should start at the carbonyl oxygen atom
B) The curved arrow should start at the hydride, go to the hydrogen connected to oxygen and a second arrow should start from the O-H covalent bond and go to the oxygen
C) The curved arrow should start at the oxygen atom of the OH
D) There should be one more arrow
Correct Answer

verified
Correct Answer
verified
Multiple Choice
What is the conjugate base of phosphoric acid?
A) HPO42-
B) H2PO4-
C) HPO32-
D) PO43-
E) None of these
Correct Answer

verified
Correct Answer
verified
Essay
Draw the conjugate base of CH3CH2NH2.
Correct Answer

verified
Correct Answer
verified
Essay
Determine if H2O is a suitable reagent to protonate the following compound. Explain why. 
Correct Answer

verified
No.
The base and the conjugate...View Answer
Show Answer
Correct Answer
verified
The base and the conjugate...
View Answer
Showing 21 - 40 of 126
Related Exams